Does neoadjuvant chemotherapy increase breast conservation in operable breast cancer: an Egyptian experience
- PMID: 22275993
- PMCID: PMC3223990
- DOI: 10.3332/ecancer.2008.104
Does neoadjuvant chemotherapy increase breast conservation in operable breast cancer: an Egyptian experience
Abstract
Introduction: The role of adjuvant chemotherapy in breast cancer is well established, as are its indications. Likewise, the role of neoadjuvant chemotherapy in locally advanced breast cancer is well established. The use of neoadjuvant chemotherapy in operable breast cancer has only recently become of interest to researchers.
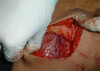
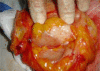
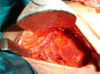
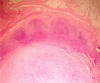
Patients and methods: This study included 34 cases of operable breast cancer that were given four cycles of neoadjuvant chemotherapy in the form of FEC100 then subjected to surgery. The surgery done was either breast conserving surgery or modified radical mastectomy. All patients completed the treatment regimen and no patients were excluded from the study. All surgical specimens were studied pathologically for chemotherapy effect.
Results: An overall objective response was observed in 70.6% of the patients. Seven patients (20.6%) experienced a clinical complete response (cCR), 17 patients (50.0%) had partial response, nine patients (26.5%) had no change of their disease and only one patient had disease progression. Of the seven patients who had a cCR, only four patients (11.8%) had pathologic complete response (pCR), while pCR for the whole group was 14.7%(5/34). Tumour size of more than 2 cm was observed in 28 patients (82.4%) at time of presentation, while tumour size of 2 cm or less was seen in six patients (17.6%) only. After completion of the course of chemotherapy, 23 patients (67.6%) were observed to have tumours of 2 cm or less that allowed for less extensive resections. Twenty-three patients underwent breast conservative surgery (67.6%) while modified radical mastectomy was performed in 11 patients (32.4%).
Conclusion: The use of neoadjuvant chemotherapy in operable breast cancer in this study was associated with tumour and axillary downstaging, which increased the proportion of cases undergoing breast conservation, with acceptable side effects and reasonable cost. During the limited follow-up time of this study no loco regional recurrences were recorded and one distant treatment failure was recorded. Its impact if any on overall or disease-free survival was not addressed in this study. Larger multi-centre randomized studies with a long follow-up are needed to compare the overall and disease-free survival benefit of this treatment modality, especially in different subtypes stratified by pathological response.
Figures
Similar articles
-
Possibility of conservative local treatment after combined chemotherapy and preoperative irradiation for locally advanced noninflammatory breast cancer.Int J Radiat Oncol Biol Phys. 1996 Mar 15;34(5):1019-28. doi: 10.1016/0360-3016(95)02207-4. Int J Radiat Oncol Biol Phys. 1996. PMID: 8600084
-
Conservative surgery after neoadjuvant chemotherapy in patients with operable breast cancer.Ann Ital Chir. 2018;89:290. Ann Ital Chir. 2018. PMID: 30352955
-
Neoadjuvant treatment with paclitaxel and epirubicin in invasive breast cancer: a phase II study.Clin Drug Investig. 2006;26(12):691-701. doi: 10.2165/00044011-200626120-00003. Clin Drug Investig. 2006. PMID: 17274676 Clinical Trial.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
-
Neoadjuvant chemotherapy in breast cancer.Br J Surg. 2005 Jan;92(1):14-23. doi: 10.1002/bjs.4840. Br J Surg. 2005. PMID: 15635596 Review.
Cited by
-
An update on the management of breast cancer in Africa.Infect Agent Cancer. 2017 Feb 14;12:13. doi: 10.1186/s13027-017-0124-y. eCollection 2017. Infect Agent Cancer. 2017. PMID: 28228841 Free PMC article. Review.
-
Pathological responses and survival outcomes in patients with locally advanced breast cancer after neoadjuvant chemotherapy: a single-institute experience.J Egypt Natl Canc Inst. 2021 Dec 14;33(1):39. doi: 10.1186/s43046-021-00096-y. J Egypt Natl Canc Inst. 2021. PMID: 34905125 Free PMC article.
References
-
- Fisher B, Gunduz N, Saffer E. Influence of the interval between primary tumour removal and chemotherapy on kinetics and growth metastases. Cancer Res. 1983;43:1488–92. - PubMed
LinkOut - more resources
Full Text Sources
Medical
