The protective effect of amifostine on ultraviolet B-exposed xeroderma pigmentosum mice
- PMID: 22276030
- PMCID: PMC3234034
- DOI: 10.3332/ecancer.2010.176
The protective effect of amifostine on ultraviolet B-exposed xeroderma pigmentosum mice
Abstract
Background: Amifostine is a pharmaceutical agent that is used clinically to counteract the side-effects of chemotherapy and radiotherapy. It acts as a free radical scavenger that protects against harmful DNA cross-linking. The purpose of this study was to determine the effect of amifostine on the development of skin cancer in xeroderma pigmentosum (XP) mice exposed to ultraviolet B radiation (UVB).
Methods: Twenty-five XP mice were equally divided into five groups. Group 1 (control) received no amifostine and no UVB exposure. Group 2 also received no amifostine, but was exposed to UVB at a dose of 200 mJ/cm(2) every other day. The remaining groups were subjected to the same irradiation, but were given amifostine at a dose of 50 mg/kg (group 3), 100 mg/kg (group 4), or 200 mg/kg (group 5) immediately prior to each exposure.
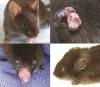
Results: No tumours were seen in the control group. The animals in group 2 (no amifostine) developed squamous cell carcinoma (SCC) at 3.5-4.5 months (mean 3.9 months). Groups 3 and 4 (low- and medium-dose amifostine) developed SCC at 4.0-7.0 months (mean 5.3 months), representing a statistically significant delay in tumour presentation (p = 0.04). An even greater delay was seen in group 5 (high-dose amifostine), which developed SCC at 7.0-9.0 months (mean 8.5 months, p < 0.001 versus groups 3 and 4). Ocular keratitis developed in all animals except the unexposed controls and the high-dose treatment group.
Conclusion: Treatment with amifostine significantly delays the onset of skin cancer and prevents ocular keratitis in UVB-exposed XP mice.
Figures
Similar articles
-
Amifostine in simultaneous radiochemotherapy of advanced head and neck cancer.Semin Radiat Oncol. 2002 Jan;12(1 Suppl 1):4-13. doi: 10.1053/srao.2002.31356. Semin Radiat Oncol. 2002. PMID: 11917277 Clinical Trial.
-
Ultraviolet radiation-induced impairment of tumor rejection is enhanced in xeroderma pigmentosum a gene-deficient mice.J Invest Dermatol. 2005 Jun;124(6):1313-7. doi: 10.1111/j.0022-202X.2005.23717.x. J Invest Dermatol. 2005. PMID: 15955109
-
Incidence of xeroderma pigmentosum in Larkana, Pakistan: a 7-year study.Br J Dermatol. 2005 Mar;152(3):545-51. doi: 10.1111/j.1365-2133.2004.06311.x. Br J Dermatol. 2005. PMID: 15787826
-
Amifostine: chemotherapeutic and radiotherapeutic protective effects.Expert Opin Pharmacother. 2001 Mar;2(3):479-89. doi: 10.1517/14656566.2.3.479. Expert Opin Pharmacother. 2001. PMID: 11336600 Review.
-
Amifostine: an update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome.Drugs. 2001;61(5):641-84. doi: 10.2165/00003495-200161050-00012. Drugs. 2001. PMID: 11368288 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
