A novel PAN/apple domain-containing protein from Toxoplasma gondii: characterization and receptor identification
- PMID: 22276154
- PMCID: PMC3261864
- DOI: 10.1371/journal.pone.0030169
A novel PAN/apple domain-containing protein from Toxoplasma gondii: characterization and receptor identification
Abstract
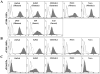
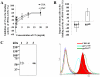
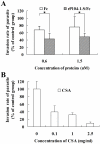
Toxoplasma gondii is an intracellular parasite that invades nucleated cells, causing toxoplasmosis in humans and animals worldwide. The extremely wide range of hosts susceptible to T. gondii is thought to be the result of interactions between T. gondii ligands and receptors on its target cells. In this study, a host cell-binding protein from T. gondii was characterized, and one of its receptors was identified. P104 (GenBank Access. No. CAJ20677) is 991 amino acids in length, containing a putative 26 amino acid signal peptide and 10 PAN/apple domains, and shows low homology to other identified PAN/apple domain-containing molecules. A 104-kDa host cell-binding protein was detected in the T. gondii lysate. Immunofluorescence assays detected P104 at the apical end of extracellular T. gondii. An Fc-fusion protein of the P104 N-terminus, which contains two PAN/apple domains, showed strong affinity for the mammalian and insect cells evaluated. This binding was not related to protein-protein or protein-lipid interactions, but to a protein-glycosaminoglycan (GAG) interaction. Chondroitin sulfate (CS), a kind of GAG, was shown to be involved in adhesion of the Fc-P104 N-terminus fusion protein to host cells. These results suggest that P104, expressed at the apical end of the extracellular parasite, may function as a ligand in the attachment of T. gondii to CS or other receptors on the host cell, facilitating invasion by the parasite.
Conflict of interest statement
Figures




Similar articles
-
Characterization and binding analysis of a microneme adhesive repeat domain-containing protein from Toxoplasma gondii.Parasitol Int. 2014 Apr;63(2):381-8. doi: 10.1016/j.parint.2013.12.006. Epub 2013 Dec 18. Parasitol Int. 2014. PMID: 24361285
-
A Toxoplasma gondii thioredoxin with cell adhesion and antioxidant function.Front Cell Infect Microbiol. 2024 Aug 15;14:1404120. doi: 10.3389/fcimb.2024.1404120. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39211799 Free PMC article.
-
Identification of host proteins interacting with the integrin-like A domain of Toxoplasma gondii micronemal protein MIC2 by yeast-two-hybrid screening.Parasit Vectors. 2014 Nov 26;7:543. doi: 10.1186/s13071-014-0543-1. Parasit Vectors. 2014. PMID: 25423901 Free PMC article.
-
Armed and dangerous: Toxoplasma gondii uses an arsenal of secretory proteins to infect host cells.Parasitol Int. 1999 Mar;48(1):1-10. doi: 10.1016/s1383-5769(98)00042-7. Parasitol Int. 1999. PMID: 11269320 Review.
-
Toxoplasma gondii: microneme protein MIC2.Int J Biochem Cell Biol. 2005 Nov;37(11):2266-72. doi: 10.1016/j.biocel.2005.06.006. Int J Biochem Cell Biol. 2005. PMID: 16084754 Review.
Cited by
-
In silico identification and ex vivo evaluation of Toxoplasma gondii peptides restricted to HLA-A*02, HLA-A*24 and HLA-B*35 alleles in human PBMC from a Colombian population.Med Microbiol Immunol. 2024 Dec 31;214(1):5. doi: 10.1007/s00430-024-00815-x. Med Microbiol Immunol. 2024. PMID: 39738923 Free PMC article.
-
Characterization of a Toxoplasma gondii calcium calmodulin-dependent protein kinase homolog.Parasit Vectors. 2016 Jul 21;9(1):405. doi: 10.1186/s13071-016-1676-1. Parasit Vectors. 2016. PMID: 27444499 Free PMC article.
-
The unique architecture and function of cellulose-interacting proteins in oomycetes revealed by genomic and structural analyses.BMC Genomics. 2012 Nov 9;13:605. doi: 10.1186/1471-2164-13-605. BMC Genomics. 2012. PMID: 23140525 Free PMC article.
-
Global proteomic analysis of the oocyst/sporozoite of Toxoplasma gondii reveals commitment to a host-independent lifestyle.BMC Genomics. 2013 Mar 15;14:183. doi: 10.1186/1471-2164-14-183. BMC Genomics. 2013. PMID: 23496850 Free PMC article.
-
The Cryptosporidium parvum C-Type Lectin CpClec Mediates Infection of Intestinal Epithelial Cells via Interactions with Sulfated Proteoglycans.Infect Immun. 2016 Apr 22;84(5):1593-1602. doi: 10.1128/IAI.01410-15. Print 2016 May. Infect Immun. 2016. PMID: 26975991 Free PMC article.
References
-
- Remington JS, McLeod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington J, Klein G, Wilson C, Baker C, editors. Infectious diseases of the fetus and newborn infant. Philadelphia: WB Saunders; 2006. pp. 947–1091.
-
- Kaufman HE, Geisler PH. The Hematologic Toxicity of Pyrimethamine (Daraprim) in Man. Arch Ophthalmol. 1960;64:140–146. - PubMed
-
- Fohl LM, Roos DS. Fitness effects of DHFR-TS mutations associated with pyrimethamine resistance in apicomplexan parasites. Mol Microbiol. 2003;50:1319–1327. - PubMed
-
- Peyron F. When are we going to celebrate the centenary of the discovery of efficient treatment for congenital toxoplasmosis? Mem Inst Oswaldo Cruz. 2009;104:316–319. - PubMed
-
- Bülow R, Boothroyd JC. Protection of mice from fatal Toxoplasma gondii infection by immunization with p30 antigen in liposomes. J Immunol. 1991;147:3496–3500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

