Angiotensin II infusion induces marked diaphragmatic skeletal muscle atrophy
- PMID: 22276172
- PMCID: PMC3262800
- DOI: 10.1371/journal.pone.0030276
Angiotensin II infusion induces marked diaphragmatic skeletal muscle atrophy
Abstract
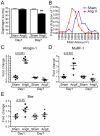
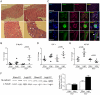
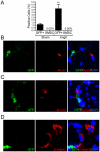
Advanced congestive heart failure (CHF) and chronic kidney disease (CKD) are characterized by increased angiotensin II (Ang II) levels and are often accompanied by significant skeletal muscle wasting that negatively impacts mortality and morbidity. Both CHF and CKD patients have respiratory muscle dysfunction, however the potential effects of Ang II on respiratory muscles are unknown. We investigated the effects of Ang II on diaphragm muscle in FVB mice. Ang II induced significant diaphragm muscle wasting (18.7±1.6% decrease in weight at one week) and reduction in fiber cross-sectional area. Expression of the E3 ubiquitin ligases atrogin-1 and muscle ring finger-1 (MuRF-1) and of the pro-apoptotic factor BAX was increased after 24 h of Ang II infusion (4.4±0.3 fold, 3.1±0.5 fold and 1.6±0.2 fold, respectively, compared to sham infused control) suggesting increased muscle protein degradation and apoptosis. In Ang II infused animals, there was significant regeneration of injured diaphragm muscles at 7 days as indicated by an increase in the number of myofibers with centralized nuclei and high expression of embryonic myosin heavy chain (E-MyHC, 11.2±3.3 fold increase) and of the satellite cell marker M-cadherin (59.2±22.2% increase). Furthermore, there was an increase in expression of insulin-like growth factor-1 (IGF-1, 1.8±0.3 fold increase) in Ang II infused diaphragm, suggesting the involvement of IGF-1 in diaphragm muscle regeneration. Bone-marrow transplantation experiments indicated that although there was recruitment of bone-marrow derived cells to the injured diaphragm in Ang II infused mice (267.0±74.6% increase), those cells did not express markers of muscle stem cells or regenerating myofibers. In conclusion, Ang II causes marked diaphragm muscle wasting, which may be important for the pathophysiology of respiratory muscle dysfunction and cachexia in conditions such as CHF and CKD.
Conflict of interest statement
Figures
References
-
- Mancini DM, Henson D, LaManca J, Levine S. Respiratory muscle function and dyspnea in patients with chronic congestive heart failure. Circulation. 1992;86:909–918. - PubMed
-
- Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation. 2001;103:2153–2158. - PubMed
-
- Strassburg S, Springer J, Anker SD. Muscle wasting in cardiac cachexia. Int J Biochem Cell Biol. 2005;37:1938–1947. - PubMed
-
- van Hees HW, van der Heijden HF, Ottenheijm CA, Heunks LM, Pigmans CJ, et al. Diaphragm single-fiber weakness and loss of myosin in congestive heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H819–828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

