Aristolochic acid I induced autophagy extenuates cell apoptosis via ERK 1/2 pathway in renal tubular epithelial cells
- PMID: 22276178
- PMCID: PMC3262826
- DOI: 10.1371/journal.pone.0030312
Aristolochic acid I induced autophagy extenuates cell apoptosis via ERK 1/2 pathway in renal tubular epithelial cells
Abstract
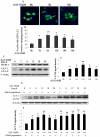
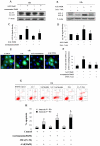
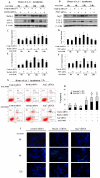
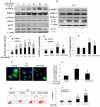
Autophagy is a lysosomal degradation pathway that is essential for cell survival and tissue homeostasis. However, limited information is available about autophagy in aristolochic acid (AA) nephropathy. In this study, we investigated the role of autophagy and related signaling pathway during progression of AAI-induced injury to renal tubular epithelial cells (NRK52E cells). The results showed that autophagy in NRK52E cells was detected as early as 3-6 hrs after low dose of AAI (10 µM) exposure as indicated by an up-regulated expression of LC3-II and Beclin 1 proteins. The appearance of AAI-induced punctated staining of autophagosome-associated LC3-II upon GFP-LC3 transfection in NRK52E cells provided further evidence for autophagy. However, cell apoptosis was not detected until 12 hrs after AAI treatment. Blockade of autophagy with Wortmannin or 3-Methyladenine (two inhibitors of phosphoinositede 3-kinases) or small-interfering RNA knockdown of Beclin 1 or Atg7 sensitized the tubular cells to apoptosis. Treatment of NRK52E cells with AAI caused a time-dependent increase in extracellular signal-regulated kinase 1 and 2 (ERK1/2) activity, but not c-Jun N-terminal kinase (JNK) and p38. Pharmacological inhibition of ERK1/2 phosphorylation with U0126 resulted in a decreased AAI-induced autophagy that was accompanied by an increased apoptosis. Taken together, our study demonstrated for the first time that autophagy occurred earlier than apoptosis during AAI-induced tubular epithelial cell injury. Autophagy induced by AAI via ERK1/2 pathway might attenuate apoptosis, which may provide a protective mechanism for cell survival under AAI-induced pathological condition.
Conflict of interest statement
Figures

References
-
- Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793:664–673. - PubMed
-
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. - PubMed
-
- Li J, Zhang L, Jiang Z, Shu B, Li F, et al. Toxicities of aristolochic acid I and aristololactam I in cultured renal epithelial cells. Toxicol In Vitro. 2010;24:1092–1097. - PubMed
-
- Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;294:F777–F787. - PubMed
-
- Pallet N, Bouvier N, Legendre C, Gilleron J, Codogno P, et al. Autophagy protects renal tubular cells against cyclosporine toxicity. Autophagy. 2008;4:783–791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

