Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation
- PMID: 22276188
- PMCID: PMC3262819
- DOI: 10.1371/journal.pone.0030380
Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation
Abstract
Background: Nitrite is a nitric oxide (NO) metabolite in tissues and blood, which can be converted to NO under hypoxia to facilitate tissue perfusion. Although nitrite is known to cause vasodilation following its reduction to NO, the effect of nitrite on platelet activity remains unclear. In this study, the effect of nitrite and nitrite+erythrocytes, with and without deoxygenation, on platelet activity was investigated.
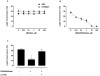
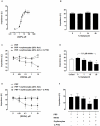
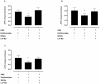
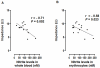
Methodology/finding: Platelet aggregation was studied in platelet-rich plasma (PRP) and PRP+erythrocytes by turbidimetric and impedance aggregometry, respectively. In PRP, DEANONOate inhibited platelet aggregation induced by ADP while nitrite had no effect on platelets. In PRP+erythrocytes, the inhibitory effect of DEANONOate on platelets decreased whereas nitrite at physiologic concentration (0.1 µM) inhibited platelet aggregation and ATP release. The effect of nitrite+erythrocytes on platelets was abrogated by C-PTIO (a membrane-impermeable NO scavenger), suggesting an NO-mediated action. Furthermore, deoxygenation enhanced the effect of nitrite as observed from a decrease of P-selectin expression and increase of the cGMP levels in platelets. The ADP-induced platelet aggregation in whole blood showed inverse correlations with the nitrite levels in whole blood and erythrocytes.
Conclusion: Nitrite alone at physiological levels has no effect on platelets in plasma. Nitrite in the presence of erythrocytes inhibits platelets through its reduction to NO, which is promoted by deoxygenation. Nitrite may have role in modulating platelet activity in the circulation, especially during hypoxia.
Conflict of interest statement
Figures
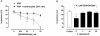
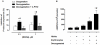
Similar articles
-
A flow cytometric analysis of the inhibition of platelet reactivity due to nitrite reduction by deoxygenated erythrocytes.PLoS One. 2014 Mar 18;9(3):e92435. doi: 10.1371/journal.pone.0092435. eCollection 2014. PLoS One. 2014. PMID: 24642865 Free PMC article.
-
Phosphorylated vasodilator-stimulated phosphoprotein (P-VASPSer239) in platelets is increased by nitrite and partially deoxygenated erythrocytes.PLoS One. 2018 Mar 5;13(3):e0193747. doi: 10.1371/journal.pone.0193747. eCollection 2018. PLoS One. 2018. PMID: 29505609 Free PMC article.
-
Role of inorganic nitrate and nitrite in driving nitric oxide-cGMP-mediated inhibition of platelet aggregation in vitro and in vivo.J Thromb Haemost. 2014 Nov;12(11):1880-9. doi: 10.1111/jth.12711. Epub 2014 Oct 1. J Thromb Haemost. 2014. PMID: 25163536
-
Effects of nitrite and far-red light on coagulation.Nitric Oxide. 2021 Feb 1;107:11-18. doi: 10.1016/j.niox.2020.11.005. Epub 2020 Nov 30. Nitric Oxide. 2021. PMID: 33271226 Free PMC article.
-
Platelet inhibition and increased phosphorylated vasodilator-stimulated phosphoprotein following sodium nitrite inhalation.Nitric Oxide. 2017 Jun 1;66:10-16. doi: 10.1016/j.niox.2017.02.008. Epub 2017 Feb 21. Nitric Oxide. 2017. PMID: 28235634
Cited by
-
Effect of storage on levels of nitric oxide metabolites in platelet preparations.Transfusion. 2013 Mar;53(3):637-44. doi: 10.1111/j.1537-2995.2012.03777.x. Epub 2012 Jul 15. Transfusion. 2013. PMID: 22804724 Free PMC article.
-
A flow cytometric analysis of the inhibition of platelet reactivity due to nitrite reduction by deoxygenated erythrocytes.PLoS One. 2014 Mar 18;9(3):e92435. doi: 10.1371/journal.pone.0092435. eCollection 2014. PLoS One. 2014. PMID: 24642865 Free PMC article.
-
Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex.Free Radic Biol Med. 2013 Dec;65:1521-1532. doi: 10.1016/j.freeradbiomed.2013.06.031. Epub 2013 Jun 24. Free Radic Biol Med. 2013. PMID: 23806384 Free PMC article. Clinical Trial.
-
Nitric oxide formation versus scavenging: the red blood cell balancing act.J Physiol. 2012 Oct 15;590(20):4993-5000. doi: 10.1113/jphysiol.2012.234906. Epub 2012 Jun 11. J Physiol. 2012. PMID: 22687616 Free PMC article. Review.
-
Reply to comments on 'vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway'.Br J Clin Pharmacol. 2013 Jun;75(6):1543-4. doi: 10.1111/bcp.12037. Br J Clin Pharmacol. 2013. PMID: 23163339 Free PMC article. No abstract available.
References
-
- Gruetter CA, Gruetter DY, Lyon JE, Kadowitz PJ, Ignarro LJ. Relationship between cyclic guanosine 3′:5′-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981;219:181–186. - PubMed
-
- Garthwaite J. New insight into the functioning of nitric oxide-receptive guanylyl cyclase: physiological and pharmacological implications. Mol Cell Biochem. 2010;334:221–232. - PubMed
-
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. - PubMed
-
- Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

