Purification and activity testing of the full-length YycFGHI proteins of Staphylococcus aureus
- PMID: 22276191
- PMCID: PMC3262814
- DOI: 10.1371/journal.pone.0030403
Purification and activity testing of the full-length YycFGHI proteins of Staphylococcus aureus
Abstract
Background: The YycFG two-component regulatory system (TCS) of Staphylococcus aureus represents the only essential TCS that is almost ubiquitously distributed in gram-positive bacteria with a low G+C-content. YycG (WalK/VicK) is a sensor histidine-kinase and YycF (WalR/VicR) is the cognate response regulator. Both proteins play an important role in the biosynthesis of the cell envelope and mutations in these proteins have been involved in development of vancomycin and daptomycin resistance.
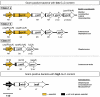
Methodology/principal findings: Here we present high yield expression and purification of the full-length YycG and YycF proteins as well as of the auxiliary proteins YycH and YycI of Staphylococcus aureus. Activity tests of the YycG kinase and a mutated version, that harbours an Y306N exchange in its cytoplasmic PAS domain, in a detergent-micelle-model and a phosholipid-liposome-model showed kinase activity (autophosphorylation and phosphoryl group transfer to YycF) only in the presence of elevated concentrations of alkali salts. A direct comparison of the activity of the kinases in the liposome-model indicated a higher activity of the mutated YycG kinase. Further experiments indicated that YycG responds to fluidity changes in its microenvironment.
Conclusions/significance: The combination of high yield expression, purification and activity testing of membrane and membrane-associated proteins provides an excellent experimental basis for further protein-protein interaction studies and for identification of all signals received by the YycFGHI system.
Conflict of interest statement
Figures






Similar articles
-
YycH regulates the activity of the essential YycFG two-component system in Bacillus subtilis.J Bacteriol. 2005 Aug;187(15):5419-26. doi: 10.1128/JB.187.15.5419-5426.2005. J Bacteriol. 2005. PMID: 16030236 Free PMC article.
-
Biochemical characterization of the first essential two-component signal transduction system from Staphylococcus aureus and Streptococcus pneumoniae.J Mol Microbiol Biotechnol. 2003;5(4):252-60. doi: 10.1159/000071077. J Mol Microbiol Biotechnol. 2003. PMID: 12867749
-
YycH and YycI interact to regulate the essential YycFG two-component system in Bacillus subtilis.J Bacteriol. 2007 Apr;189(8):3280-9. doi: 10.1128/JB.01936-06. Epub 2007 Feb 16. J Bacteriol. 2007. PMID: 17307850 Free PMC article.
-
Tearing down the wall: peptidoglycan metabolism and the WalK/WalR (YycG/YycF) essential two-component system.Adv Exp Med Biol. 2008;631:214-28. doi: 10.1007/978-0-387-78885-2_15. Adv Exp Med Biol. 2008. PMID: 18792692 Review.
-
Daptomycin: mechanisms of action and resistance, and biosynthetic engineering.Curr Opin Chem Biol. 2009 Apr;13(2):144-51. doi: 10.1016/j.cbpa.2009.02.031. Epub 2009 Mar 19. Curr Opin Chem Biol. 2009. PMID: 19303806 Review.
Cited by
-
Genomic and Long-Term Transcriptomic Imprints Related to the Daptomycin Mechanism of Action Occurring in Daptomycin- and Methicillin-Resistant Staphylococcus aureus Under Daptomycin Exposure.Front Microbiol. 2020 Aug 14;11:1893. doi: 10.3389/fmicb.2020.01893. eCollection 2020. Front Microbiol. 2020. PMID: 32922373 Free PMC article.
-
Involvement of WalK (VicK) phosphatase activity in setting WalR (VicR) response regulator phosphorylation level and limiting cross-talk in Streptococcus pneumoniae D39 cells.Mol Microbiol. 2012 Nov;86(3):645-60. doi: 10.1111/mmi.12006. Epub 2012 Sep 27. Mol Microbiol. 2012. PMID: 23013245 Free PMC article.
-
Defective pgsA contributes to increased membrane fluidity and cell wall thickening in Staphylococcus aureus with high-level daptomycin resistance.mSphere. 2024 Jun 25;9(6):e0011524. doi: 10.1128/msphere.00115-24. Epub 2024 May 16. mSphere. 2024. PMID: 38752757 Free PMC article.
-
The First Paenibacillus larvae Bacteriophage Endolysin (PlyPl23) with High Potential to Control American Foulbrood.PLoS One. 2015 Jul 13;10(7):e0132095. doi: 10.1371/journal.pone.0132095. eCollection 2015. PLoS One. 2015. PMID: 26167894 Free PMC article.
-
Shape Follows Function: Gastrointestinal Signals for Enterococcal Colonization.Trends Mol Med. 2019 Jun;25(6):464-466. doi: 10.1016/j.molmed.2019.04.011. Epub 2019 May 17. Trends Mol Med. 2019. PMID: 31109795 Free PMC article.
References
-
- Ng WL, Winkler ME. Singular structures and operon organizations of essential two-component systems in species of Streptococcus. Microbiology. 2004;150:3096–3098. - PubMed
-
- Dubrac S, Bisicchia P, Devine K, Msadek T. A matter of life and death: cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol Microbiol. 2008;70:1307–1322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

