Dedifferentiation of foetal CNS stem cells to mesendoderm-like cells through an EMT process
- PMID: 22276221
- PMCID: PMC3262838
- DOI: 10.1371/journal.pone.0030759
Dedifferentiation of foetal CNS stem cells to mesendoderm-like cells through an EMT process
Abstract
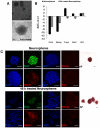
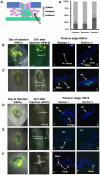
Tissue-specific stem cells are considered to have a limited differentiation potential. Recently, this notion was challenged by reports that showed a broader differentiation potential of neural stem cells, in vitro and in vivo, although the molecular mechanisms that regulate plasticity of neural stem cells are unknown. Here, we report that neural stem cells derived from mouse embryonic cortex respond to Lif and serum in vitro and undergo epithelial to mesenchymal transition (EMT)-mediated dedifferentiation process within 48 h, together with transient upregulation of pluripotency markers and, more notably, upregulation of mesendoderm genes, Brachyury (T) and Sox17. These induced putative mesendoderm cells were injected into early gastrulating chick embryos, which revealed that they integrated more efficiently into mesoderm and endoderm lineages compared to non-induced cells. We also found that TGFβ and Jak/Stat pathways are necessary but not sufficient for the induction of mesendodermal phenotype in neural stem cells. These results provide insights into the regulation of plasticity of neural stem cells through EMT. Dissecting the regulatory pathways involved in these processes may help to gain control over cell fate decisions.
Conflict of interest statement
Figures





References
-
- Gilbert SF. Developmental Biology. 2006.
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

