Topical androgen antagonism promotes cutaneous wound healing without systemic androgen deprivation by blocking β-catenin nuclear translocation and cross-talk with TGF-β signaling in keratinocytes
- PMID: 22276587
- PMCID: PMC5461922
- DOI: 10.1111/j.1524-475X.2011.00757.x
Topical androgen antagonism promotes cutaneous wound healing without systemic androgen deprivation by blocking β-catenin nuclear translocation and cross-talk with TGF-β signaling in keratinocytes
Abstract
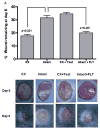
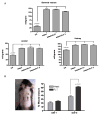
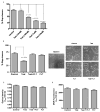
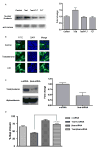
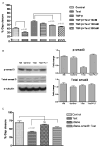
Orchidectomy in rodents and lower testosterone levels in men are associated with improved cutaneous wound healing. However, due to the adverse effects on skeletal and sexual tissues, systemic androgen blockade is not a viable therapeutic intervention. Accordingly, we tested the hypothesis that topical application of an androgen antagonist would elicit accelerated wound healing without systemic androgen antagonism. Full-thickness cutaneous wounds were created on adult C57BL6/J mice. Daily topical application of androgen receptor antagonist, flutamide, resulted in improved gap closure similar to orchiectomized controls and faster than orchidectomized mice treated with topical testosterone. In vivo data showed that the effects of androgen antagonism on wound closure primarily accelerate keratinocytes migration without effecting wound contraction. Consequently, mechanisms of testosterone action on reepithelialization were investigated in vitro by scratch wounding assays in confluent keratinocytes. Testosterone inhibited keratinocyte migration and this effect was in part mediated through promotion of nuclear translocation of β-catenin and by attenuating transforming growth factor-β (TGF-β) signaling through β-catenin. The link between Wnt and TGF beta signaling was confirmed by blocking β-catenin and by following TGF-β-induced transcription of a luciferase reporter gene. Together, these data show that blockade of β-catenin can, as a potential target for novel therapeutic interventions, accelerate cutaneous wound healing.
© 2012 by the Wound Healing Society.
Figures


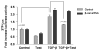
References
-
- Ashcroft GS, Dodsworth J, van Boxtel E, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MW. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat Med. 1997;3:1209–15. - PubMed
-
- Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119 (Pt 4):722–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

