Intermolecular Ritter-type C-H amination of unactivated sp3 carbons
- PMID: 22276612
- PMCID: PMC3277284
- DOI: 10.1021/ja212020b
Intermolecular Ritter-type C-H amination of unactivated sp3 carbons
Abstract
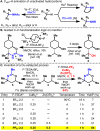
Intermolecular Ritter-type C-H amination of unactivated sp(3) carbons has been developed. This new reaction proceeds under mild conditions using readily available reagents and an inexpensive source of nitrogen (acetonitrile). A broad scope of substrates can be aminated with this method since many functional groups are tolerated. This reaction also allows for the direct, innate C-H amination of a variety of hydrocarbons such as cyclohexane without the need of prefunctionalization or installation of a directing group.
Figures


References
-
- Barton DHR, editor. Reason and Imagination: Reflections on Research in Organic Chemistry: Selected Papers of Derek H. R. Barton. World Scientific; River Edge, NJ: 1996.
-
- Breslow R, Baldwin SW. J. Am. Chem. Soc. 1970;92:732–734.
-
- Corey EJ, Hertler WR. J. Am. Chem. Soc. 1958;80:2903–2904.
-
-
For selected recent reviews, see: Collet F, Lescot C, Dauban P. Chem. Soc. Rev. 2011;40:1926–1936.. Zalatan DN, Du Bois J. Top. Curr. Chem. 2010;292:347–378.. Collet F, Dodd RH, Dauban P. Chem. Commun. 2009:5061–5074.. Díaz-Requejo MM, Pérez PJ. Chem. Rev. 2008;108:3379–3394..
-
-
- Hofmann AW. Ber. 1883;16:558–560.
- Hofmann AW. Ber. 1885;18:5–23.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

