Hyaluronan synthase mediates dye translocation across liposomal membranes
- PMID: 22276637
- PMCID: PMC3331846
- DOI: 10.1186/1471-2091-13-2
Hyaluronan synthase mediates dye translocation across liposomal membranes
Abstract
Background: Hyaluronan (HA) is made at the plasma membrane and secreted into the extracellular medium or matrix by phospolipid-dependent hyaluronan synthase (HAS), which is active as a monomer. Since the mechanism by which HA is translocated across membranes is still unresolved, we assessed the presence of an intraprotein pore within HAS by adding purified Streptococcus equisimilis HAS (SeHAS) to liposomes preloaded with the fluorophore Cascade Blue (CB).
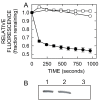
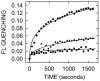
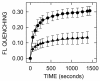
Results: CB translocation (efflux) was not observed with mock-purified material from empty vector control E. coli membranes, but was induced by SeHAS, purified from membranes, in a time- and dose-dependent manner. CB efflux was eliminated or greatly reduced when purified SeHAS was first treated under conditions that inhibit enzyme activity: heating, oxidization or cysteine modification with N-ethylmaleimide. Reduced CB efflux also occurred with SeHAS K48E or K48F mutants, in which alteration of K48 within membrane domain 2 causes decreased activity and HA product size. The above results used liposomes containing bovine cardiolipin (BCL). An earlier study testing many synthetic lipids found that the best activating lipid for SeHAS is tetraoleoyl cardiolipin (TO-CL) and that, in contrast, tetramyristoyl cardiolipin (TM-CL) is an inactivating lipid (Weigel et al, J. Biol. Chem. 281, 36542, 2006). Consistent with the effects of these CL species on SeHAS activity, CB efflux was more than 2-fold greater in liposomes made with TO-CL compared to TM-CL.
Conclusions: The results indicate the presence of an intraprotein pore in HAS and support a model in which HA is translocated to the exterior by HAS itself.
© 2012 Medina et al; licensee BioMed Central Ltd.
Figures


Similar articles
-
Hyaluronan Synthase: The Mechanism of Initiation at the Reducing End and a Pendulum Model for Polysaccharide Translocation to the Cell Exterior.Int J Cell Biol. 2015;2015:367579. doi: 10.1155/2015/367579. Epub 2015 Sep 10. Int J Cell Biol. 2015. PMID: 26472958 Free PMC article. Review.
-
Phospholipid dependence and liposome reconstitution of purified hyaluronan synthase.J Biol Chem. 2006 Dec 1;281(48):36542-51. doi: 10.1074/jbc.M606529200. Epub 2006 Sep 19. J Biol Chem. 2006. PMID: 16984914
-
Molecular cloning, expression, and characterization of the authentic hyaluronan synthase from group C Streptococcus equisimilis.J Biol Chem. 1997 Dec 19;272(51):32539-46. doi: 10.1074/jbc.272.51.32539. J Biol Chem. 1997. PMID: 9405467
-
The streptococcal hyaluronan synthases are inhibited by sulfhydryl-modifying reagents, but conserved cysteine residues are not essential for enzyme function.J Biol Chem. 2002 Apr 19;277(16):13943-51. doi: 10.1074/jbc.M110638200. Epub 2002 Jan 17. J Biol Chem. 2002. PMID: 11799120
-
Purification and lipid dependence of the recombinant hyaluronan synthases from Streptococcus pyogenes and Streptococcus equisimilis.J Biol Chem. 1999 Feb 12;274(7):4239-45. doi: 10.1074/jbc.274.7.4239. J Biol Chem. 1999. PMID: 9933623
Cited by
-
Hyaluronan synthase polymerizing activity and control of product size are discrete enzyme functions that can be uncoupled by mutagenesis of conserved cysteines.Glycobiology. 2012 Oct;22(10):1302-10. doi: 10.1093/glycob/cws102. Epub 2012 Jun 27. Glycobiology. 2012. PMID: 22745284 Free PMC article.
-
Fluorescence resonance energy transfer (FRET) and proximity ligation assays reveal functionally relevant homo- and heteromeric complexes among hyaluronan synthases HAS1, HAS2, and HAS3.J Biol Chem. 2015 May 1;290(18):11479-90. doi: 10.1074/jbc.M115.640581. Epub 2015 Mar 20. J Biol Chem. 2015. PMID: 25795779 Free PMC article.
-
ABCC5 is required for cAMP-mediated hindgut invagination in sea urchin embryos.Development. 2015 Oct 15;142(20):3537-48. doi: 10.1242/dev.126144. Epub 2015 Sep 22. Development. 2015. PMID: 26395488 Free PMC article.
-
Hyaluronan synthase 1: a mysterious enzyme with unexpected functions.Front Immunol. 2015 Feb 5;6:43. doi: 10.3389/fimmu.2015.00043. eCollection 2015. Front Immunol. 2015. PMID: 25699059 Free PMC article. Review.
-
Hyaluronan Synthase: The Mechanism of Initiation at the Reducing End and a Pendulum Model for Polysaccharide Translocation to the Cell Exterior.Int J Cell Biol. 2015;2015:367579. doi: 10.1155/2015/367579. Epub 2015 Sep 10. Int J Cell Biol. 2015. PMID: 26472958 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

