Release, neuronal effects and removal of extracellular β-nicotinamide adenine dinucleotide (β-NAD⁺) in the rat brain
- PMID: 22276961
- PMCID: PMC3270379
- DOI: 10.1111/j.1460-9568.2011.07957.x
Release, neuronal effects and removal of extracellular β-nicotinamide adenine dinucleotide (β-NAD⁺) in the rat brain
Abstract
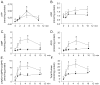
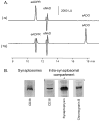
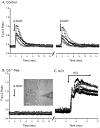
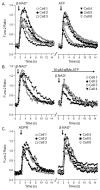
Recent evidence supports an emerging role of β-nicotinamide adenine dinucleotide (β-NAD(+) ) as a novel neurotransmitter and neuromodulator in the peripheral nervous system -β-NAD(+) is released in nerve-smooth muscle preparations and adrenal chromaffin cells in a manner characteristic of a neurotransmitter. It is currently unclear whether this holds true for the CNS. Using a small-chamber superfusion assay and high-sensitivity high-pressure liquid chromatography techniques, we demonstrate that high-K(+) stimulation of rat forebrain synaptosomes evokes overflow of β-NAD(+) , adenosine 5'-triphosphate, and their metabolites adenosine 5'-diphosphate (ADP), adenosine 5'-monophosphate, adenosine, ADP-ribose (ADPR) and cyclic ADPR. The high-K(+) -evoked overflow of β-NAD(+) is attenuated by cleavage of SNAP-25 with botulinum neurotoxin A, by inhibition of N-type voltage-dependent Ca(2+) channels with ω-conotoxin GVIA, and by inhibition of the proton gradient of synaptic vesicles with bafilomycin A1, suggesting that β-NAD(+) is likely released via vesicle exocytosis. Western analysis demonstrates that CD38, a multifunctional protein that metabolizes β-NAD(+) , is present on synaptosomal membranes and in the cytosol. Intact synaptosomes degrade β-NAD(+) . 1,N (6) -etheno-NAD, a fluorescent analog of β-NAD(+) , is taken by synaptosomes and this uptake is attenuated by authentic β-NAD(+) , but not by the connexin 43 inhibitor Gap 27. In cortical neurons local applications of β-NAD(+) cause rapid Ca(2+) transients, likely due to influx of extracellular Ca(2+) . Therefore, rat brain synaptosomes can actively release, degrade and uptake β-NAD(+) , and β-NAD(+) can stimulate postsynaptic neurons, all criteria needed for a substance to be considered a candidate neurotransmitter in the brain.
© 2012 The Authors. European Journal of Neuroscience © 2012 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Conflict of interest statement
Authors declare no conflict of interest.
Figures



Similar articles
-
Adenosine 5-diphosphate-ribose is a neural regulator in primate and murine large intestine along with β-NAD(+).J Physiol. 2012 Apr 15;590(8):1921-41. doi: 10.1113/jphysiol.2011.222414. Epub 2012 Feb 20. J Physiol. 2012. PMID: 22351627 Free PMC article.
-
Storage and secretion of beta-NAD, ATP and dopamine in NGF-differentiated rat pheochromocytoma PC12 cells.Eur J Neurosci. 2009 Sep;30(5):756-68. doi: 10.1111/j.1460-9568.2009.06869.x. Epub 2009 Aug 27. Eur J Neurosci. 2009. PMID: 19712094 Free PMC article.
-
beta-NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle.Am J Physiol Renal Physiol. 2006 Feb;290(2):F486-95. doi: 10.1152/ajprenal.00314.2005. Epub 2005 Sep 27. Am J Physiol Renal Physiol. 2006. PMID: 16189287
-
Neuronal and extraneuronal release of ATP and NAD(+) in smooth muscle.IUBMB Life. 2012 Oct;64(10):817-24. doi: 10.1002/iub.1076. Epub 2012 Sep 3. IUBMB Life. 2012. PMID: 22941916 Free PMC article. Review.
-
The purinergic neurotransmitter revisited: a single substance or multiple players?Pharmacol Ther. 2014 Nov;144(2):162-91. doi: 10.1016/j.pharmthera.2014.05.012. Epub 2014 Jun 2. Pharmacol Ther. 2014. PMID: 24887688 Free PMC article. Review.
Cited by
-
NADomics: Measuring NAD+ and Related Metabolites Using Liquid Chromatography Mass Spectrometry.Life (Basel). 2021 May 31;11(6):512. doi: 10.3390/life11060512. Life (Basel). 2021. PMID: 34073099 Free PMC article. Review.
-
A unifying mechanism of ketogenic diet action: The multiple roles of nicotinamide adenine dinucleotide.Epilepsy Res. 2020 Nov;167:106469. doi: 10.1016/j.eplepsyres.2020.106469. Epub 2020 Sep 14. Epilepsy Res. 2020. PMID: 33038721 Free PMC article. Review.
-
The human NAD metabolome: Functions, metabolism and compartmentalization.Crit Rev Biochem Mol Biol. 2015;50(4):284-97. doi: 10.3109/10409238.2015.1028612. Epub 2015 Apr 2. Crit Rev Biochem Mol Biol. 2015. PMID: 25837229 Free PMC article. Review.
-
Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFRα-positive cells, and interstitial cells of Cajal in the murine colon.Neurogastroenterol Motil. 2013 Sep;25(9):e609-20. doi: 10.1111/nmo.12174. Epub 2013 Jun 30. Neurogastroenterol Motil. 2013. PMID: 23809506 Free PMC article.
-
Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5.Nutrients. 2022 Jan 22;14(3):484. doi: 10.3390/nu14030484. Nutrients. 2022. PMID: 35276844 Free PMC article. Review.
References
-
- Amara SG, Kuhar MJ. Neurotransmitter Transporters: Recent Progress. Annual Review of Neuroscience. 1993;16:73–93. - PubMed
-
- Billington RA, Travelli C, Ercolano E, Galli U, Roman CB, Grolla AA, Canonico PL, Condorelli F, Genazzani AA. Characterization of NAD Uptake in Mammalian Cells. J Biol Chem. 2008;283:6367–6374. - PubMed
-
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–163. - PubMed
-
- Bobalova J, Mutafova-Yambolieva VN. Membrane-bound and releasable nucleotidase activities: differences in canine mesenteric artery and vein. Clin Exp Pharmacol Physiol. 2003;30:194–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

