Effect of Saccharomyces cerevisiae var. Boulardii and β-galactomannan oligosaccharide on porcine intestinal epithelial and dendritic cells challenged in vitro with Escherichia coli F4 (K88)
- PMID: 22277078
- PMCID: PMC3305624
- DOI: 10.1186/1297-9716-43-4
Effect of Saccharomyces cerevisiae var. Boulardii and β-galactomannan oligosaccharide on porcine intestinal epithelial and dendritic cells challenged in vitro with Escherichia coli F4 (K88)
Abstract
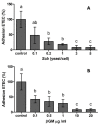
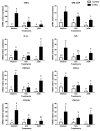
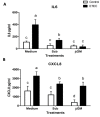
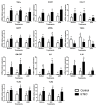
Probiotic and prebiotics, often called "immune-enhancing" feed additives, are believed to deal with pathogens, preventing the need of an immune response and reducing tissue damage. In this study, we investigated if a recently developed β-galactomannan (βGM) had a similar protective role compared to Saccharomyces cerevisiae var. Boulardii (Scb), a proven probiotic, in the context of enterotoxigenic Escherichia coli (ETEC) infection. ETEC causes inflammation, diarrhea and intestinal damage in piglets, resulting in large economic loses worldwide. We observed that Scb and βGM products inhibited in vitro adhesion of ETEC on cell surface of porcine intestinal IPI-2I cells. Our data showed that Scb and βGM decreased the mRNA ETEC-induced gene expression of pro-inflammatory cytokines TNF-α, IL-6, GM-CSF and chemokines CCL2, CCL20 and CXCL8 on intestinal IPI-2I. Furthermore, we investigated the putative immunomodulatory role of Scb and βGM on porcine monocyte-derived dendritic cells (DCs) per se and under infection conditions. We observed a slight up-regulation of mRNA for TNF-α and CCR7 receptor after co-incubation of DC with Scb and βGM. However, no differences were found in DC activation upon ETEC infection and Scb or βGM co-culture. Therefore, our results indicate that, similar to probiotic Scb, prebiotic βGM may protect intestinal epithelial cells against intestinal pathogens. Finally, although these products may modulate DC activation, their effect under ETEC challenge conditions remains to be elucidated.
Figures
Similar articles
-
β-Galactomannan and Saccharomyces cerevisiae var. boulardii modulate the immune response against Salmonella enterica serovar Typhimurium in porcine intestinal epithelial and dendritic cells.Clin Vaccine Immunol. 2012 Mar;19(3):368-76. doi: 10.1128/CVI.05532-11. Epub 2012 Feb 1. Clin Vaccine Immunol. 2012. PMID: 22301691 Free PMC article.
-
Administration of probiotics influences F4 (K88)-positive enterotoxigenic Escherichia coli attachment and intestinal cytokine expression in weaned pigs.Vet Res. 2011 May 23;42(1):69. doi: 10.1186/1297-9716-42-69. Vet Res. 2011. PMID: 21605377 Free PMC article.
-
Saccharomyces cerevisiae decreases inflammatory responses induced by F4+ enterotoxigenic Escherichia coli in porcine intestinal epithelial cells.Vet Immunol Immunopathol. 2011 May 15;141(1-2):133-8. doi: 10.1016/j.vetimm.2011.01.018. Epub 2011 Feb 26. Vet Immunol Immunopathol. 2011. PMID: 21354630
-
Saccharomyces cerevisiae modulates immune gene expressions and inhibits ETEC-mediated ERK1/2 and p38 signaling pathways in intestinal epithelial cells.PLoS One. 2011 Apr 4;6(4):e18573. doi: 10.1371/journal.pone.0018573. PLoS One. 2011. PMID: 21483702 Free PMC article.
-
Saccharomyces cerevisiae boulardii accelerates intestinal microbiota maturation and is correlated with increased secretory IgA production in neonatal dairy calves.Front Microbiol. 2023 Sep 19;14:1129250. doi: 10.3389/fmicb.2023.1129250. eCollection 2023. Front Microbiol. 2023. PMID: 37795296 Free PMC article. Review.
Cited by
-
Effects of Dietary β-Mannanase Supplementation on Growth Performance, Apparent Total Tract Digestibility, Intestinal Integrity, and Immune Responses in Weaning Pigs.Animals (Basel). 2020 Apr 17;10(4):703. doi: 10.3390/ani10040703. Animals (Basel). 2020. PMID: 32316523 Free PMC article.
-
Gut microbiome and resistome characterization of pigs treated with commonly used post-weaning diarrhea treatments.Anim Microbiome. 2024 May 3;6(1):24. doi: 10.1186/s42523-024-00307-6. Anim Microbiome. 2024. PMID: 38702766 Free PMC article.
-
Oligosaccharide structure determines prebiotic role of β-galactomannan against Salmonella enterica ser. Typhimurium in vitro.Gut Microbes. 2013 Jan-Feb;4(1):72-5. doi: 10.4161/gmic.22728. Epub 2012 Nov 8. Gut Microbes. 2013. PMID: 23137964 Free PMC article.
-
Expression of recombination antimicrobial protein PIL22-PBD-2 in Pichia pastoris and verification of its biological function in vitro.Vet Res. 2025 Mar 7;56(1):52. doi: 10.1186/s13567-024-01428-1. Vet Res. 2025. PMID: 40055823 Free PMC article.
-
Effects of prebiotics on immune system and cytokine expression.Med Microbiol Immunol. 2017 Feb;206(1):1-9. doi: 10.1007/s00430-016-0481-y. Epub 2016 Oct 4. Med Microbiol Immunol. 2017. PMID: 27704207 Review.
References
-
- World Health Organisation. The Medical Impact of the use of antimicrobials in food animals. 13-17 October; Berlin, Germany. 1997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

