The role of melatonin as an antioxidant in the follicle
- PMID: 22277103
- PMCID: PMC3296634
- DOI: 10.1186/1757-2215-5-5
The role of melatonin as an antioxidant in the follicle
Abstract
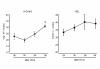
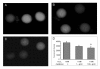
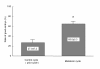
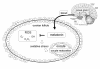
Melatonin (N-acetyl-5-methoxytryptamine) is secreted during the dark hours at night by pineal gland, and it regulates a variety of important central and peripheral actions related to circadian rhythms and reproduction. It has been believed that melatonin regulates ovarian function by the regulation of gonadotropin release in the hypothalamus-pituitary gland axis via its specific receptors. In addition to the receptor mediated action, the discovery of melatonin as a direct free radical scavenger has greatly broadened the understanding of melatonin's mechanisms which benefit reproductive physiology. Higher concentrations of melatonin have been found in human preovulatory follicular fluid compared to serum, and there is growing evidence of the direct effects of melatonin on ovarian function especially oocyte maturation and embryo development. Many scientists have focused on the direct role of melatonin on oocyte maturation and embryo development as an anti-oxidant to reduce oxidative stress induced by reactive oxygen species, which are produced during ovulation process. The beneficial effects of melatonin administration on oocyte maturation and embryo development have been confirmed by in vitro and in vivo experiments in animals. This review also discusses the first application of melatonin to the clinical treatment of infertile women and confirms that melatonin administration reduces intrafollicular oxidative damage and increase fertilization rates. This review summarizes our recent works and new findings related to the reported beneficial effects of melatonin on reproductive physiology in its role as a reducer of oxidative stress, especially on oocyte maturation and embryo development.
Figures
References
-
- Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166(12 Pt 2):S4–8. - PubMed
LinkOut - more resources
Full Text Sources
Medical

