GRK2: multiple roles beyond G protein-coupled receptor desensitization
- PMID: 22277298
- PMCID: PMC3294176
- DOI: 10.1016/j.tips.2011.12.003
GRK2: multiple roles beyond G protein-coupled receptor desensitization
Abstract
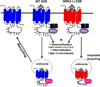
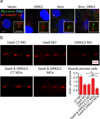
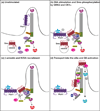
G protein-coupled receptor kinases (GRKs) regulate numerous G protein-coupled receptors (GPCRs) by phosphorylating the intracellular domain of the active receptor, resulting in receptor desensitization and internalization. GRKs also regulate GPCR trafficking in a phosphorylation-independent manner via direct protein-protein interactions. Emerging evidence suggests that GRK2, the most widely studied member of this family of kinases, modulates multiple cellular responses in various physiological contexts by either phosphorylating non-receptor substrates or interacting directly with signaling molecules. In this review, we discuss traditional and newly discovered roles of GRK2 in receptor internalization and signaling as well as its impact on non-receptor substrates. We also discuss novel exciting roles of GRK2 in the regulation of dopamine receptor signaling and in the activation and trafficking of the atypical GPCR, Smoothened (Smo).
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict: The authors declare no conflict of interest.
Figures
References
-
- Takeda S, et al. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. - PubMed
-
- Pierce KL, et al. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. - PubMed
-
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. - PubMed
-
- Pitcher JA, et al. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

