[18F]CFT synthesis and binding to monoamine transporters in rats
- PMID: 22277306
- PMCID: PMC3299608
- DOI: 10.1186/2191-219X-2-3
[18F]CFT synthesis and binding to monoamine transporters in rats
Abstract
Background: We present the electrophilic synthesis of [18F]2β-carbomethoxy-3β-(4-fluoro)tropane [[18F]CFT] and the pharmacological specificity and selectivity of [18F]CFT for monoamine transporters in the brain and peripheral organs of rats. The human radiation dose is extrapolated from the animal data.
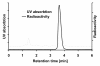
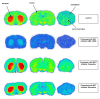
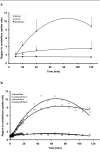
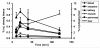
Methods: [18F]CFT was synthesized by electrophilic fluorination of a stannylated precursor by using post-target-produced [18F]F2 as a fluorinating agent. The ex vivo 18F-activity biodistribution of [18F]CFT in the brain of rats was studied by autoradiography. The binding of [18F]CFT to the monoamine transporters was studied using in vivo blocking experiments with dopamine transporter [DAT], norepinephrine transporter [NET], or serotonin transporter [SERT] inhibitors. In vivo animal positron emission tomography was used as a comparative method to determine tracer kinetics. Human radiation dose was assessed using OLINDA software.
Results: The radiochemical yield of [18F]CFT from the initial [18F]F-, decay corrected to the end of bombardment, was 3.2 ± 1.0%. The specific activity [SA] was 14.5 ± 3.4 GBq/μmol, decay corrected to the end of synthesis. Radiochemical purity exceeded 99%. DAT-specific binding was found in the striatum, locus coeruleus, and pancreas. NET-specific binding was found in the locus coeruleus. SERT-specific binding was not found in any of the studied organs. Effective dose equivalent [EDE] estimated for the standard human model was 12.8 μSv/MBq. Effective dose [ED] was 9.17 μSv/MBq.
Conclusions: Post-target-produced high-SA [18F]F2 was used to incorporate18F directly into the phenyl ring of [18F]CFT. The final product had high radiochemical and chemical purities and a high SA for DAT and NET studies in vivo. In periphery, [18F]CFT showed a specific uptake in the pancreas. EDE and ED corresponded well with other18F-radioligands.
Figures


Similar articles
-
Comparison of 2beta-carbomethoxy-3beta-(4-[18F]fluorophenyl)tropane and N-(3-[18F]fluoropropyl)-2beta-carbomethoxy-3beta-(4-fluorophenyl)nortropane, tracers for imaging dopamine transporter in rat.Mol Imaging Biol. 2010 Jun;12(3):269-77. doi: 10.1007/s11307-009-0278-0. Epub 2009 Dec 1. Mol Imaging Biol. 2010. PMID: 19949983
-
18F-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters.Nucl Med Biol. 2000 Jan;27(1):1-12. doi: 10.1016/s0969-8051(99)00080-3. Nucl Med Biol. 2000. PMID: 10755640
-
Synthesis, radiosynthesis, and biological evaluation of carbon-11 and iodine-123 labeled 2beta-carbomethoxy-3beta-[4'-((Z)-2-haloethenyl)phenyl]tropanes: candidate radioligands for in vivo imaging of the serotonin transporter.J Med Chem. 2004 Feb 26;47(5):1122-35. doi: 10.1021/jm030384e. J Med Chem. 2004. PMID: 14971892
-
Synthesis and characterization of iodine-123 labeled 2beta-carbomethoxy-3beta-(4'-((Z)-2-iodoethenyl)phenyl)nortropane. A ligand for in vivo imaging of serotonin transporters by single-photon-emission tomography.J Med Chem. 2003 Mar 13;46(6):925-35. doi: 10.1021/jm0100180. J Med Chem. 2003. PMID: 12620070
-
In vivo characterization of a novel norepinephrine transporter PET tracer [18F]NS12137 in adult and immature Sprague-Dawley rats.Theranostics. 2019 Jan 1;9(1):11-19. doi: 10.7150/thno.29740. eCollection 2019. Theranostics. 2019. PMID: 30662550 Free PMC article.
Cited by
-
Radiosynthesis and validation of ¹⁸F-FP-CMT, a phenyltropane with superior properties for imaging the dopamine transporter in living brain.J Cereb Blood Flow Metab. 2014 Jul;34(7):1148-56. doi: 10.1038/jcbfm.2014.63. Epub 2014 Apr 9. J Cereb Blood Flow Metab. 2014. PMID: 24714035 Free PMC article.
-
Comparison of high and low molar activity TSPO tracer [18F]F-DPA in a mouse model of Alzheimer's disease.J Cereb Blood Flow Metab. 2020 May;40(5):1012-1020. doi: 10.1177/0271678X19853117. Epub 2019 May 29. J Cereb Blood Flow Metab. 2020. PMID: 31142224 Free PMC article.
-
Exploring the nigrostriatal and digestive interplays in Parkinson's disease using dynamic total-body [11C]CFT PET/CT.Eur J Nucl Med Mol Imaging. 2024 Jul;51(8):2271-2282. doi: 10.1007/s00259-024-06638-5. Epub 2024 Feb 23. Eur J Nucl Med Mol Imaging. 2024. PMID: 38393375
-
The influence of genetic variants on striatal dopamine transporter and D2 receptor binding after TBI.J Cereb Blood Flow Metab. 2014 Aug;34(8):1328-39. doi: 10.1038/jcbfm.2014.87. Epub 2014 May 21. J Cereb Blood Flow Metab. 2014. PMID: 24849661 Free PMC article.
-
Development of (18)F-labeled radiotracers for neuroreceptor imaging with positron emission tomography.Neurosci Bull. 2014 Oct;30(5):777-811. doi: 10.1007/s12264-014-1460-6. Epub 2014 Aug 29. Neurosci Bull. 2014. PMID: 25172118 Free PMC article. Review.
References
-
- Wong DF, Yung B, Dannals RF, Shaya EK, Ravert HT, Chen CA, Chan B, Folio T, Scheffel U, Ricaurte GA, Neumeyer JL, Wagner HN Jr, Kuhar MJ. In vivo imaging of baboon and human dopamine transporters by positron emission tomography using [11C]WIN 35,428. Synapse. 1993;15:130–142. doi: 10.1002/syn.890150205. - DOI - PubMed
-
- Varrone A, Steiger C, Schou M, Takano A, Finnema SJ, Guilloteau D, Gulyás B, Halldin C. In vitro autoradiography and in vivo evaluation in cynomolgus monkey of [18F]FE-PE2I, a new dopamine transporter PET radioligand. Synapse. 2009;10:871–880. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

