Repeated Radionuclide therapy in metastatic paraganglioma leading to the highest reported cumulative activity of 131I-MIBG
- PMID: 22277577
- PMCID: PMC3277473
- DOI: 10.1186/1748-717X-7-8
Repeated Radionuclide therapy in metastatic paraganglioma leading to the highest reported cumulative activity of 131I-MIBG
Abstract
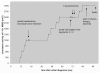
131I-MIBG therapy for neuroendocrine tumours may be dose limited. The common range of applied cumulative activities is 10-40 GBq. We report the uneventful cumulative administration of 111 GBq (= 3 Ci) 131I-MIBG in a patient with metastatic paraganglioma. Ten courses of 131I-MIBG therapy were given within six years, accomplishing symptomatic, hormonal and tumour responses with no serious adverse effects. Chemotherapy with cisplatin/vinblastine/dacarbazine was the final treatment modality with temporary control of disease, but eventually the patient died of progression. The observed cumulative activity of 131I-MIBG represents the highest value reported to our knowledge, and even though 12.6 GBq of 90Y-DOTATOC were added intermediately, no associated relevant bone marrow, hepatic or other toxicity were observed. In an individual attempt to palliate metastatic disease high cumulative activity alone should not preclude the patient from repeat treatment.
Figures

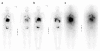
Similar articles
-
Treatment of metastatic carcinoid tumours, phaeochromocytoma, paraganglioma and medullary carcinoma of the thyroid with (131)I-meta-iodobenzylguanidine [(131)I-mIBG].Clin Endocrinol (Oxf). 2001 Jul;55(1):47-60. doi: 10.1046/j.1365-2265.2001.01309.x. Clin Endocrinol (Oxf). 2001. PMID: 11453952
-
Functional imaging as an aid to decision-making in metastatic paraganglioma.Br J Radiol. 2001 Mar;74(879):266-9. doi: 10.1259/bjr.74.879.740266. Br J Radiol. 2001. PMID: 11338105
-
Therapeutic effectiveness of iodine-131 MIBG metastases of a nonsecreting paraganglioma.J Nucl Med. 1988 Dec;29(12):2008-13. J Nucl Med. 1988. PMID: 3193214
-
High-Specific-Activity-131I-MIBG versus 177Lu-DOTATATE Targeted Radionuclide Therapy for Metastatic Pheochromocytoma and Paraganglioma.Clin Cancer Res. 2021 Jun 1;27(11):2989-2995. doi: 10.1158/1078-0432.CCR-20-3703. Epub 2021 Mar 8. Clin Cancer Res. 2021. PMID: 33685867 Free PMC article. Review.
-
The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients.J Endocrinol Invest. 1997 Dec;20(11):648-58. doi: 10.1007/BF03348026. J Endocrinol Invest. 1997. PMID: 9492103 Review.
Cited by
-
A phase I clinical trial for [131I]meta-iodobenzylguanidine therapy in patients with refractory pheochromocytoma and paraganglioma.Sci Rep. 2019 May 20;9(1):7625. doi: 10.1038/s41598-019-43880-6. Sci Rep. 2019. PMID: 31110198 Free PMC article. Clinical Trial.
References
-
- Garaventa A, Guerra P, Arrighini A, Bertolazzi L, Bestagno M, De Bernardi B, Lanino E, Villavecchia GP, Claudiani F. Treatment of advanced neuroblastoma with I-131 meta-iodobenzylguanidine. Cancer. 1991;67:922–928. doi: 10.1002/1097-0142(19910215)67:4<922::AID-CNCR2820670411>3.0.CO;2-D. - DOI - PubMed
-
- Mukherjee JJ, Kaltsas GA, Islam N, Plowman PN, Foley R, Hikmat J, Britton KE, Jenkins PJ, Chew SL, Monson JP, Besser GM, Grossman AB. Treatment of metastatic carcinoid tumours, phaeochromocytoma, paraganglioma and medullary carcinoma of the thyroid with (131)I-meta-iodobenzylguanidine [(131)I-mIBG] Clin Endocrinol (Oxf) 2001;55:47–60. doi: 10.1046/j.1365-2265.2001.01309.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

