Development and validation of a repharsed phase- HPLC method for simultaneous determination of rosiglitazone and glimepiride in combined dosage forms and human plasma
- PMID: 22277722
- PMCID: PMC3292994
- DOI: 10.1186/1752-153X-6-9
Development and validation of a repharsed phase- HPLC method for simultaneous determination of rosiglitazone and glimepiride in combined dosage forms and human plasma
Abstract
Background: Rosiglitazone (ROZ) and glimepiride (GLM) are antidiabetic agents used in the treatment of type 2 diabetes mellitus. A survey of the literature reveals that only one spectrophotometric method has been reported for the simultaneous determination of ROS and GLM in pharmaceutical preparations. However the reported method suffers from the low sensitivity, for this reason, our target was to develop a simple sensitive HPLC method for the simultaneous determination of ROZ and GLM in their combined dosage forms and plasma.
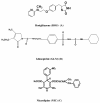
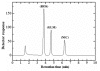
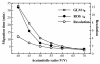
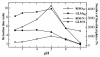
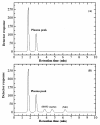
Results: A simple reversed phase high performance liquid chromatographic (RP-HPLC) method was developed and validated for the simultaneous determination of Rosiglitazone (ROS) and Glimepiride (GLM) in combined dosage forms and human plasma. The separation was achieved using a 150 mm × 4.6 mm i.d., 5 μm particle size Symmetry® C18 column. Mobile phase containing a mixture of acetonitrile and 0.02 M phosphate buffer of pH 5 (60: 40, V/V) was pumped at a flow rate of 1 mL/min. UV detection was performed at 235 nm using nicardipine as an internal standard. The method was validated for accuracy, precision, specificity, linearity, and sensitivity. The developed and validated method was successfully used for quantitative analysis of Avandaryl™ tablets. The chromatographic analysis time was approximately 7 min per sample with complete resolution of ROS (tR = 3.7 min.), GLM (tR = 4.66 min.), and nicardipine (tR, 6.37 min). Validation studieswas performed according to ICH Guidelines revealed that the proposed method is specific, rapid, reliable and reproducible. The calibration plots were linear over the concentration ranges 0.10-25 μg/mL and 0.125-12.5 μg/mL with LOD of 0.04 μg/mL for both compounds and limits of quantification 0.13 and 0.11 μg/mL for ROS and GLM respectively.
Conclusion: The suggested method was successfully applied for the simultaneous analysis of the studied drugs in their co-formulated tablets and human plasma. The mean percentage recoveries in Avandaryl™ tablets were 100.88 ± 1.14 and 100.31 ± 1.93 for ROS and GLM respectively. Statistical comparison of the results with those of the reference method revealed good agreement and proved that there were no significant difference in the accuracy and precision between the two methods respectively. The interference likely to be introduced from some co-administered drugs such as glibenclamide, gliclazide, metformine, pioglitazone and nateglinide was investigated.
Figures
Similar articles
-
Micellar liquid chromatographic method for the simultaneous determination of Levofloxacin and Ambroxol in combined tablets: Application to biological fluids.Chem Cent J. 2013 Oct 1;7(1):162. doi: 10.1186/1752-153X-7-162. Chem Cent J. 2013. PMID: 24079576 Free PMC article.
-
Development and Validation of Stability-Indicating RP-HPLC Method for Simultaneous Determination of Metformin HCl and Glimepiride in Fixed-Dose Combination.Anal Chem Insights. 2016 Mar 13;11:13-20. doi: 10.4137/ACI.S38137. eCollection 2016. Anal Chem Insights. 2016. PMID: 26997866 Free PMC article.
-
RP-LC simultaneous quantitation of co-administered drugs for (non-insulin dependent) diabetic mellitus induced dyslipidemia in active pharmaceutical ingredient, pharmaceutical formulations and human serum with UV-detector.Clin Chim Acta. 2013 Oct 21;425:54-61. doi: 10.1016/j.cca.2013.06.020. Epub 2013 Jul 7. Clin Chim Acta. 2013. PMID: 23838368
-
Stability Indicating RP-HPLC Method for the Simultaneous Determination of Atorvastatin Calcium, Metformin Hydrochloride, and Glimepiride in Bulk and Combined Tablet Dosage Form.Int Sch Res Notices. 2014 Oct 29;2014:754695. doi: 10.1155/2014/754695. eCollection 2014. Int Sch Res Notices. 2014. PMID: 27433531 Free PMC article.
-
Simultaneous determination of metformin, nateglinide and gliclazide in pharmaceutical preparations using micellar liquid chromatography.Int J Biomed Sci. 2012 Jun;8(2):144-51. Int J Biomed Sci. 2012. PMID: 23675267 Free PMC article.
Cited by
-
Rapid Analysis of Glibenclamide Using an Environmentally Benign Stability-Indicating RP-HPLC Method.Iran J Pharm Res. 2014 Summer;13(3):863-72. Iran J Pharm Res. 2014. PMID: 25276186 Free PMC article.
-
An integrated Taguchi and response surface methodological approach for the optimization of an HPLC method to determine glimepiride in a supersaturatable self-nanoemulsifying formulation.Saudi Pharm J. 2016 Jan;24(1):92-103. doi: 10.1016/j.jsps.2015.03.004. Epub 2015 Mar 23. Saudi Pharm J. 2016. PMID: 26903773 Free PMC article.
-
Improved Transmucosal Delivery of Glimepiride via Unidirectional Release Buccal Film Loaded With Vitamin E TPGS-Based Nanocarrier.Dose Response. 2020 Jul 31;18(3):1559325820945164. doi: 10.1177/1559325820945164. eCollection 2020 Jul-Sep. Dose Response. 2020. PMID: 32782450 Free PMC article.
References
-
- AVANDARYL® (rosiglitazone maleate and glimepiride) Tablets. http://www.medicineonline.com/drugs/A/4200/AVANDARYL-rosiglitazone-malea...
-
- Gomes P, Steppe M. First-derivative spectrophotometry in the analysis of rosiglitazone in coated tablets. J AOAC Int. 2006;89:1296–1299. - PubMed
-
- Walash MI, El-Brashy A, El-Enany N, Kamel ME. Spectrofluorimetric and spectrophotometric determination of rosiglitazone maleate in pharmaceutical preparations and biological fluids. Pharmaceutical Chemistry Journal. 2009;43:697–709. doi: 10.1007/s11094-010-0383-z. - DOI
-
- El Sherbiny D, El-Enany N, Belal F. Voltammetric Determination of Rosiglitazone in Pharmaceutical Preparations and Human Plasma. Anal Lett. 2008;41:806–821. doi: 10.1080/00032710801935046. - DOI
LinkOut - more resources
Full Text Sources

