Incorporation of phosphate group modulates bone cell attachment and differentiation on oligo(polyethylene glycol) fumarate hydrogel
- PMID: 22277774
- PMCID: PMC3970912
- DOI: 10.1016/j.actbio.2011.12.031
Incorporation of phosphate group modulates bone cell attachment and differentiation on oligo(polyethylene glycol) fumarate hydrogel
Abstract
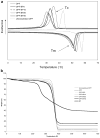
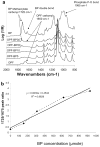
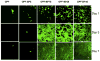
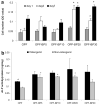
In this work, we have investigated the development of a synthetic hydrogel that contains a negatively charged phosphate group for use as a substrate for bone cell attachment and differentiation in culture. The photoreactive, phosphate-containing molecule, bis(2-(methacryloyloxy)ethyl)phosphate (BP), was incorporated into oligo(polyethylene glycol) fumarate hydrogel and the mechanical, rheological and thermal properties of the resulting hydrogels were characterized. Our results showed changes in hydrogel compression and storage moduli with incorporation of BP. The modification also resulted in decreased crystallinity as recorded by differential scanning calorimetry. Our data revealed that incorporation of BP improved attachment and differentiation of human fetal osteoblast (hFOB) cells in a dose-dependent manner. A change in surface chemistry and mineralization of the phosphate-containing surfaces verified by scanning electron microscopy and energy dispersive X-ray analysis was found to be important for hFOB cell attachment and differentiation. We also demonstrated that phosphate-containing hydrogels support attachment and differentiation of primary bone marrow stromal cells. These findings suggest that BP-modified hydrogels are capable of sustaining attachment and differentiation of both bone marrow stromal cells and osteoblasts that are critical for bone regeneration.
Copyright © 2012. Published by Elsevier Ltd.
Conflict of interest statement
A non-provisional patent has been filed for photocrosslinkable oligo(polyethylene glycol) fumarate used in this research, and this technology has been licensed to BonWrx.
Figures


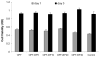



References
-
- Zouani OF, Chollet C, Guillotin B, Durrieu MC. Differentiation of pre-osteoblast cells on poly(ethylene terephthalate) grafted with RGD and/or BMPs mimetic peptides. Biomaterials. 2010;31:8245–53. - PubMed
-
- Zavgorodniy AV, Borrero-Lopez O, Hoffman M, Legeros RZ, Rohanizadeh R. Characterization of the chemically deposited hydroxyapatite coating on a titanium substrate. J Mater Sci Mater Med. 2011;22:1–9. - PubMed
-
- Zhang L, Ayukawa Y, Legeros RZ, Matsuya S, Koyano K, Ishikawa K. Tissue-response to calcium-bonded titanium surface. J Biomed Mater Res A. 2010;95:33–9. - PubMed
-
- Gandhi R, Davey JR, Mahomed NN. Hydroxyapatite coated femoral stems in primary total hip arthroplasty: a meta-analysis. J Arthroplasty. 2009;24:38–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

