Integrative responses of neurons in parabrachial nuclei to a nauseogenic gastrointestinal stimulus and vestibular stimulation in vertical planes
- PMID: 22277934
- PMCID: PMC3330766
- DOI: 10.1152/ajpregu.00680.2011
Integrative responses of neurons in parabrachial nuclei to a nauseogenic gastrointestinal stimulus and vestibular stimulation in vertical planes
Abstract
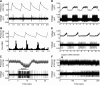
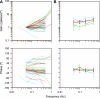
The parabrachial and adjacent Kölliker-Fuse (PBN/KF) nuclei play a key role in relaying visceral afferent inputs to the hypothalamus and limbic system and are, thus, believed to participate in generating nausea and affective responses elicited by gastrointestinal (GI) signals. In addition, the PBN/KF region receives inputs from the vestibular system and likely mediates the malaise associated with motion sickness. However, previous studies have not considered whether GI and vestibular inputs converge on the same PBN/KF neurons, and if so, whether the GI signals alter the responses of the cells to body motion. The present study, conducted in decerebrate cats, tested the hypothesis that intragastric injection of copper sulfate, which elicits emesis by irritating the stomach lining, modifies the sensitivity of PBN/KF neurons to vertical plane rotations that activate vestibular receptors. Intragastric copper sulfate produced a 70% median change in the gain of responses to vertical plane rotations of PBN/KF units, whose firing rate was modified by the administration of the compound; the response gains for 16 units increased and those for 17 units decreased. The effects were often dramatic: out of 51 neurons tested, 13 responded to the rotations only after copper sulfate was injected, whereas 10 others responded only before drug delivery. These data show that a subset of PBN/KF neurons, whose activity is altered by a nauseogenic stimulus also respond to body motion and that irritation of the stomach lining can either cause an amplification or reduction in the sensitivity of the units to vestibular inputs. The findings imply that nausea and affective responses to vestibular stimuli may be modified by the presence of emetic signals from the GI system.
Figures






Similar articles
-
Responses of neurons in the caudal medullary lateral tegmental field to visceral inputs and vestibular stimulation in vertical planes.Am J Physiol Regul Integr Comp Physiol. 2012 Nov 1;303(9):R929-40. doi: 10.1152/ajpregu.00356.2012. Epub 2012 Sep 5. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22955058 Free PMC article.
-
Effects of visceral inputs on the processing of labyrinthine signals by the inferior and caudal medial vestibular nuclei: ramifications for the production of motion sickness.Exp Brain Res. 2013 Jul;228(3):353-63. doi: 10.1007/s00221-013-3568-3. Epub 2013 May 28. Exp Brain Res. 2013. PMID: 23712685 Free PMC article.
-
Integrative responses of neurons in nucleus tractus solitarius to visceral afferent stimulation and vestibular stimulation in vertical planes.Am J Physiol Regul Integr Comp Physiol. 2011 Nov;301(5):R1380-90. doi: 10.1152/ajpregu.00361.2011. Epub 2011 Aug 10. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21832211 Free PMC article.
-
Integration of vestibular and emetic gastrointestinal signals that produce nausea and vomiting: potential contributions to motion sickness.Exp Brain Res. 2014 Aug;232(8):2455-69. doi: 10.1007/s00221-014-3937-6. Epub 2014 Apr 16. Exp Brain Res. 2014. PMID: 24736862 Free PMC article. Review.
-
Otolith and canal integration on single vestibular neurons in cats.Exp Brain Res. 2005 Jul;164(3):271-85. doi: 10.1007/s00221-005-2341-7. Epub 2005 Jul 1. Exp Brain Res. 2005. PMID: 15991028 Review.
Cited by
-
Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting.Eur J Pharmacol. 2014 Jan 5;722:55-66. doi: 10.1016/j.ejphar.2013.10.037. Epub 2013 Oct 26. Eur J Pharmacol. 2014. PMID: 24495419 Free PMC article. Review.
-
Understanding the links between vestibular and limbic systems regulating emotions.J Nat Sci Biol Med. 2017 Jan-Jun;8(1):11-15. doi: 10.4103/0976-9668.198350. J Nat Sci Biol Med. 2017. PMID: 28250668 Free PMC article. Review.
-
Physiological changes associated with copper sulfate-induced nausea and retching in felines.Front Physiol. 2023 Jan 19;14:1077207. doi: 10.3389/fphys.2023.1077207. eCollection 2023. Front Physiol. 2023. PMID: 36744037 Free PMC article.
-
Responses of neurons in the caudal medullary lateral tegmental field to visceral inputs and vestibular stimulation in vertical planes.Am J Physiol Regul Integr Comp Physiol. 2012 Nov 1;303(9):R929-40. doi: 10.1152/ajpregu.00356.2012. Epub 2012 Sep 5. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22955058 Free PMC article.
-
Feed your head: neurodevelopmental control of feeding and metabolism.Annu Rev Physiol. 2014;76:197-223. doi: 10.1146/annurev-physiol-021113-170347. Epub 2013 Nov 18. Annu Rev Physiol. 2014. PMID: 24274739 Free PMC article. Review.
References
-
- Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya H. The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett 286: 123–126, 2000 - PubMed
-
- Baird JP, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1581–R1593, 2001 - PubMed
-
- Balaban CD. Vestibular nucleus projections to the parabrachial nucleus in rabbits: Implications for vestibular influences on the autonomic nervous system. Exp Brain Res 108: 367–381, 1996 - PubMed
-
- Balaban CD, McGee DM, Zhou J, Scudder CA. Responses of primate caudal parabrachial nucleus and Kolliker-Fuse nucleus neurons to whole body rotation. J Neurophysiol 88: 3175–3193, 2002 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

