Development aggravates the severity of skeletal muscle catabolism induced by endotoxemia in neonatal pigs
- PMID: 22277935
- PMCID: PMC4499027
- DOI: 10.1152/ajpregu.00259.2011
Development aggravates the severity of skeletal muscle catabolism induced by endotoxemia in neonatal pigs
Abstract
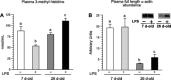
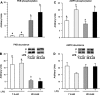
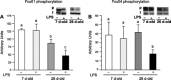
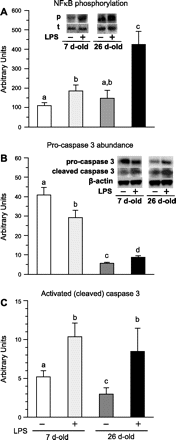
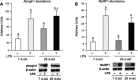
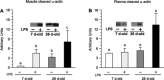
Accretion rates of muscle protein are elevated in normal neonates, but this anabolic drive decreases with maturation. As this change occurs, it is not known whether development also influences muscle protein catabolism induced by sepsis. We hypothesize that protein degradation in skeletal muscle induced by endotoxemia becomes more severe as the neonate develops. Fasted 7- and 26-day-old pigs were infused for 8 h with LPS (0 and 10 μg·kg(-1)·h(-1)), while plasma amino acids (AA), 3-methylhistidine (3-MH), and α-actin concentrations and muscle protein degradation signal activation were determined (n = 5-7/group/age). Plasma full-length α-actin was greater in 7- than 26-day-old pigs, suggesting a higher baseline protein turnover in neonatal pigs. LPS increased plasma total AA, 3-MH, and full-length and cleaved α-actin in 26- than in 7-day-old pigs. In muscle of both age groups, LPS increased AMPK and NF-κB phosphorylation, the abundances of activated caspase 3 and E-3 ligases MuRF1 and atrogin1, as well as the abundance of cleaved α-actin, suggesting activation of muscle proteolysis by endotoxin in muscle. LPS decreased Forkhead box 01 (Fox01) and Fox04 phosphorylation and increased procaspase 3 abundance in muscle of 26-day-old pigs despite the lack of effect of LPS on PKB phosphorylation. The results suggest that skeletal muscle in healthy neonatal pigs maintains high baseline degradation signal activation that cannot be enhanced by endotoxin, but as maturation advances, the effect of LPS on muscle protein catabolism manifests its severity.
Figures
Similar articles
-
Amino acids, independent of insulin, attenuate skeletal muscle autophagy in neonatal pigs during endotoxemia.Pediatr Res. 2016 Sep;80(3):448-51. doi: 10.1038/pr.2016.83. Epub 2016 Apr 11. Pediatr Res. 2016. PMID: 27064245 Free PMC article.
-
Insulin signaling in skeletal muscle and liver of neonatal pigs during endotoxemia.Pediatr Res. 2008 Nov;64(5):505-10. doi: 10.1203/PDR.0b013e318183fd4c. Pediatr Res. 2008. PMID: 18596577 Free PMC article.
-
Amino acids augment muscle protein synthesis in neonatal pigs during acute endotoxemia by stimulating mTOR-dependent translation initiation.Am J Physiol Endocrinol Metab. 2007 Nov;293(5):E1416-25. doi: 10.1152/ajpendo.00146.2007. Epub 2007 Sep 11. Am J Physiol Endocrinol Metab. 2007. PMID: 17848637
-
Roles of insulin and amino acids in the regulation of protein synthesis in the neonate.J Nutr. 1998 Feb;128(2 Suppl):347S-350S. doi: 10.1093/jn/128.2.347S. J Nutr. 1998. PMID: 9478022 Review.
-
Regulation of Muscle Growth in Early Postnatal Life in a Swine Model.Annu Rev Anim Biosci. 2019 Feb 15;7:309-335. doi: 10.1146/annurev-animal-020518-115130. Epub 2018 Nov 2. Annu Rev Anim Biosci. 2019. PMID: 30388025 Free PMC article. Review.
Cited by
-
Amino acids, independent of insulin, attenuate skeletal muscle autophagy in neonatal pigs during endotoxemia.Pediatr Res. 2016 Sep;80(3):448-51. doi: 10.1038/pr.2016.83. Epub 2016 Apr 11. Pediatr Res. 2016. PMID: 27064245 Free PMC article.
-
The Effects of Glutamine Supplementation on Liver Inflammatory Response and Protein Metabolism in Muscle of Lipopolysaccharide-Challenged Broilers.Animals (Basel). 2024 Feb 1;14(3):480. doi: 10.3390/ani14030480. Animals (Basel). 2024. PMID: 38338123 Free PMC article.
-
Regulation of protein degradation pathways by amino acids and insulin in skeletal muscle of neonatal pigs.J Anim Sci Biotechnol. 2014 Jan 17;5(1):8. doi: 10.1186/2049-1891-5-8. J Anim Sci Biotechnol. 2014. PMID: 24438646 Free PMC article.
-
Novel and disruptive biological strategies for resolving gut health challenges in monogastric food animal production.Anim Nutr. 2015 Sep;1(3):138-143. doi: 10.1016/j.aninu.2015.10.002. Epub 2015 Nov 10. Anim Nutr. 2015. PMID: 29767174 Free PMC article. Review.
-
The Sick and the Weak: Neuropathies/Myopathies in the Critically Ill.Physiol Rev. 2015 Jul;95(3):1025-109. doi: 10.1152/physrev.00028.2014. Physiol Rev. 2015. PMID: 26133937 Free PMC article. Review.
References
-
- Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004. - PubMed
-
- Cooney RN, Owens E, Slaymaker D, Vary TC. Prevention of skeletal muscle catabolism in sepsis does not impair visceral protein metabolism. Am J Physiol Endocrinol Metab 270: E621–E626, 1996. - PubMed
-
- Doucet M, Russell AP, Leger B, Debigare R, Joanisse DR, Caron MA, LeBlanc P, Maltais F. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 176: 261–269, 2007. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

