Identification of the vitamin D receptor in various cells of the mouse kidney
- PMID: 22278022
- PMCID: PMC3343313
- DOI: 10.1038/ki.2011.463
Identification of the vitamin D receptor in various cells of the mouse kidney
Abstract
The kidney is the major, if not sole, site for the production of 1α,25-dihydroxyvitamin D(3) (1,25(OH)(2)D(3)), the biologically active form of vitamin D that can stimulate calcium reabsorption in the kidney and may provide renoprotective benefits. The biological effects of 1,25(OH)(2)D(3) are mediated through a nuclear hormone receptor, known as the vitamin D receptor (VDR). It is well accepted that the VDR is present in the distal renal convoluted tubule cells; however, whether VDR is present in other kidney cell types is uncertain. Using a highly specific and sensitive anti-VDR antibody, we determined its distribution in the mouse kidney by immunohistochemistry. Our results show that the VDR is not only present in the distal but is also found in the proximal tubules, but at 24-fold lower levels. The VDR was also found in the macula densa of the juxtaglomerular apparatus, glomerular parietal epithelial cells, and podocytes. In contrast, the VDR is either very low or absent in interstitial fibroblasts, glomerular mesangial cells, and juxtaglomerular cells. Thus, identification of VDR in the proximal tubule, macula densa, and podocytes suggests that 1,25(OH)(2)D(3) plays a direct role in these cells under normal conditions.
Figures

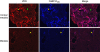

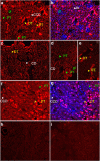



Comment in
-
Renal vitamin D receptor expression and vitamin D renoprotection.Kidney Int. 2012 May;81(10):937-939. doi: 10.1038/ki.2012.30. Kidney Int. 2012. PMID: 22543902
References
-
- Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000;80:277–313. - PubMed
-
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. - PubMed
-
- Brunette MG, Chan M, Ferriere C, et al. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature. 1978;276:287–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

