Gated regulation of CRAC channel ion selectivity by STIM1
- PMID: 22278058
- PMCID: PMC3276717
- DOI: 10.1038/nature10752
Gated regulation of CRAC channel ion selectivity by STIM1
Abstract
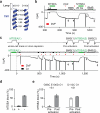
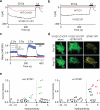
Two defining functional features of ion channels are ion selectivity and channel gating. Ion selectivity is generally considered an immutable property of the open channel structure, whereas gating involves transitions between open and closed channel states, typically without changes in ion selectivity. In store-operated Ca(2+) release-activated Ca(2+) (CRAC) channels, the molecular mechanism of channel gating by the CRAC channel activator, stromal interaction molecule 1 (STIM1), remains unknown. CRAC channels are distinguished by a very high Ca(2+) selectivity and are instrumental in generating sustained intracellular calcium concentration elevations that are necessary for gene expression and effector function in many eukaryotic cells. Here we probe the central features of the STIM1 gating mechanism in the human CRAC channel protein, ORAI1, and identify V102, a residue located in the extracellular region of the pore, as a candidate for the channel gate. Mutations at V102 produce constitutively active CRAC channels that are open even in the absence of STIM1. Unexpectedly, although STIM1-free V102 mutant channels are not Ca(2+)-selective, their Ca(2+) selectivity is dose-dependently boosted by interactions with STIM1. Similar enhancement of Ca(2+) selectivity is also seen in wild-type ORAI1 channels by increasing the number of STIM1 activation domains that are directly tethered to ORAI1 channels, or by increasing the relative expression of full-length STIM1. Thus, exquisite Ca(2+) selectivity is not an intrinsic property of CRAC channels but rather a tuneable feature that is bestowed on otherwise non-selective ORAI1 channels by STIM1. Our results demonstrate that STIM1-mediated gating of CRAC channels occurs through an unusual mechanism in which permeation and gating are closely coupled.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

