Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells
- PMID: 22278060
- PMCID: PMC3338985
- DOI: 10.1038/nature10821
Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells
Abstract
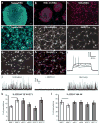
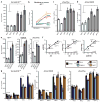
Our understanding of Alzheimer's disease pathogenesis is currently limited by difficulties in obtaining live neurons from patients and the inability to model the sporadic form of the disease. It may be possible to overcome these challenges by reprogramming primary cells from patients into induced pluripotent stem cells (iPSCs). Here we reprogrammed primary fibroblasts from two patients with familial Alzheimer's disease, both caused by a duplication of the amyloid-β precursor protein gene (APP; termed APP(Dp)), two with sporadic Alzheimer's disease (termed sAD1, sAD2) and two non-demented control individuals into iPSC lines. Neurons from differentiated cultures were purified with fluorescence-activated cell sorting and characterized. Purified cultures contained more than 90% neurons, clustered with fetal brain messenger RNA samples by microarray criteria, and could form functional synaptic contacts. Virtually all cells exhibited normal electrophysiological activity. Relative to controls, iPSC-derived, purified neurons from the two APP(Dp) patients and patient sAD2 exhibited significantly higher levels of the pathological markers amyloid-β(1-40), phospho-tau(Thr 231) and active glycogen synthase kinase-3β (aGSK-3β). Neurons from APP(Dp) and sAD2 patients also accumulated large RAB5-positive early endosomes compared to controls. Treatment of purified neurons with β-secretase inhibitors, but not γ-secretase inhibitors, caused significant reductions in phospho-Tau(Thr 231) and aGSK-3β levels. These results suggest a direct relationship between APP proteolytic processing, but not amyloid-β, in GSK-3β activation and tau phosphorylation in human neurons. Additionally, we observed that neurons with the genome of one sAD patient exhibited the phenotypes seen in familial Alzheimer's disease samples. More generally, we demonstrate that iPSC technology can be used to observe phenotypes relevant to Alzheimer's disease, even though it can take decades for overt disease to manifest in patients.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
Neurodegenerative disease: Dishing up Alzheimer's disease.Nat Rev Neurosci. 2012 Feb 15;13(3):149. doi: 10.1038/nrn3201. Nat Rev Neurosci. 2012. PMID: 22334215 No abstract available.
-
iPSCs to the rescue in Alzheimer's research.Cell Stem Cell. 2012 Mar 2;10(3):235-6. doi: 10.1016/j.stem.2012.02.011. Cell Stem Cell. 2012. PMID: 22385650
References
-
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. - PubMed
-
- Ballatore C, Lee VMY, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nature Rev Neurosci. 2007;8:663–672. - PubMed
-
- Gatz M, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. - PubMed
-
- Games D, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

