AT-1001: a high affinity and selective α3β4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats
- PMID: 22278092
- PMCID: PMC3327842
- DOI: 10.1038/npp.2011.322
AT-1001: a high affinity and selective α3β4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats
Abstract
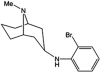
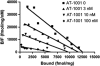
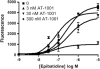
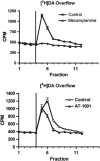
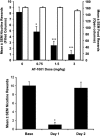
Genomic and pharmacologic data have suggested the involvement of the α3β4 subtype of nicotinic acetylcholine receptors (nAChRs) in drug seeking to nicotine and other drugs of abuse. In order to better examine this receptor subtype, we have identified and characterized the first high affinity and selective α3β4 nAChR antagonist, AT-1001, both in vitro and in vivo. This is the first reported compound with a Ki below 10 nM at α3β4 nAChR and >90-fold selectivity over the other major subtypes, the α4β2 and α7 nAChR. AT-1001 competes with epibatidine, allowing for [³H]epibatidine binding to be used for structure-activity studies, however, both receptor binding and ligand-induced Ca²⁺ flux are not strictly competitive because increasing ligand concentration produces an apparent decrease in receptor number and maximal Ca²⁺ fluorescence. AT-1001 also potently and reversibly blocks epibatidine-induced inward currents in HEK cells transfected with α3β4 nAChR. Importantly, AT-1001 potently and dose-dependently blocks nicotine self-administration in rats, without affecting food responding. When tested in a nucleus accumbens (NAcs) synaptosomal preparation, AT-1001 inhibits nicotine-induced [³H]dopamine release poorly and at significantly higher concentrations compared with mecamylamine and conotoxin MII. These results suggest that its inhibition of nicotine self-administration in rats is not directly due to a decrease in dopamine release from the NAc, and most likely involves an indirect pathway requiring α3β4 nAChR. In conclusion, our studies provide further evidence for the involvement of α3β4 nAChR in nicotine self-administration. These findings suggest the utility of this receptor as a target for smoking cessation medications, and highlight the potential of AT-1001 and congeners as clinically useful compounds.
Figures
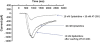

References
-
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. - PubMed
-
- Cao J, Belluzzi JD, Loughlin SE, Keyler DE, Pentel PR, Leslie FM. Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats. Neuropsychopharmacology. 2007;32:2025–2035. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

