Distinct transformation tropism exhibited by human T lymphotropic virus type 1 (HTLV-1) and HTLV-2 is the result of postinfection T cell clonal expansion
- PMID: 22278223
- PMCID: PMC3302488
- DOI: 10.1128/JVI.06900-11
Distinct transformation tropism exhibited by human T lymphotropic virus type 1 (HTLV-1) and HTLV-2 is the result of postinfection T cell clonal expansion
Abstract
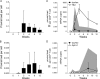
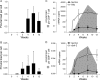
Human T lymphotropic virus type 1 (HTLV-1) and HTLV-2 are related but pathogenically distinct viruses. HTLV-1 mainly causes adult T cell leukemia, while HTLV-2 is not associated with leukemia. In vitro, HTLV-1 and HTLV-2 predominantly transform CD4(+) and CD8(+) T cells, respectively: the genetic determinant maps to the viral envelope. Herein, we investigate whether this transformation tropism occurs during initial infection or subsequently during the cellular transformation process. Since most individuals are chronically infected at the time of detection, we utilized an established rabbit model to longitudinally measure the early HTLV-1 and HTLV-2 infection and replication kinetics in purified CD4(+) and CD8(+) T cells. HTLV-1 and HTLV-2 were detected in both CD4(+) and CD8(+) T cells within 1 week postinoculation. In HTLV-1-infected rabbit CD4(+) T cells, proviral burden and tax/rex mRNA expression peaked early, and expression levels were directly proportional to each other. The late expression of the antisense transcript (Hbz or Aph-2) correlated directly with a late proviral burden peak in HTLV-1- or HTLV-2-infected rabbit CD8(+) T cells, respectively. This study provides the first in vivo evidence that these viruses do not exhibit cellular preference during initial infection. We further evaluated the transformation tropism of HTLV-1 and HTLV-2 over a 9-week period using in vitro cell growth/immortalization assays. At the early weeks, both HTLV-1 and HTLV-2 showed proportionate growth of CD4(+) and CD8(+) T cells. However, beyond week 5, the predominance of one particular T cell type emerged, supporting the conclusion that transformation tropism is a postinfection event due to selective clonal expansion over time.
Figures
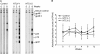


References
-
- Araujo A, Hall WW. 2004. Human T-lymphotropic virus type II and neurological disease. Ann. Neurol. 56:10–19 - PubMed
-
- Cockerell GL, Rovank J, Green PL, Chen ISY. 1996. A deletion in the proximal untranslated pX region of human T-cell leukemia virus type II decreases viral replication but not infectivity in vivo. Blood 87:1030–1035 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

