Vaccinia mature virus fusion regulator A26 protein binds to A16 and G9 proteins of the viral entry fusion complex and dissociates from mature virions at low pH
- PMID: 22278246
- PMCID: PMC3302531
- DOI: 10.1128/JVI.06081-11
Vaccinia mature virus fusion regulator A26 protein binds to A16 and G9 proteins of the viral entry fusion complex and dissociates from mature virions at low pH
Abstract
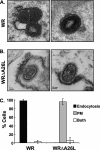
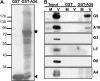
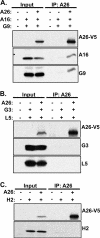
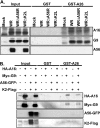
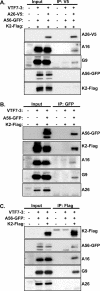
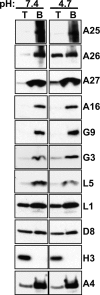
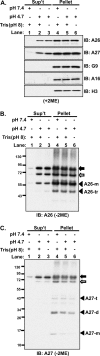
Vaccinia mature virus enters cells through either endocytosis or plasma membrane fusion, depending on virus strain and cell type. Our previous results showed that vaccinia virus mature virions containing viral A26 protein enter HeLa cells preferentially through endocytosis, whereas mature virions lacking A26 protein enter through plasma membrane fusion, leading us to propose that A26 acts as an acid-sensitive fusion suppressor for mature virus (S. J. Chang, Y. X. Chang, R. Izmailyan R, Y. L. Tang, and W. Chang, J. Virol. 84:8422-8432, 2010). In the present study, we investigated the fusion suppression mechanism of A26 protein. We found that A26 protein was coimmunoprecipitated with multiple components of the viral entry-fusion complex (EFC) in infected HeLa cells. Transient expression of viral EFC components in HeLa cells revealed that vaccinia virus A26 protein interacted directly with A16 and G9 but not with G3, L5 and H2 proteins of the EFC components. Consistently, a glutathione S-transferase (GST)-A26 fusion protein, but not GST, pulled down A16 and G9 proteins individually in vitro. Together, our results supported the idea that A26 protein binds to A16 and G9 protein at neutral pH contributing to suppression of vaccinia virus-triggered membrane fusion from without. Since vaccinia virus extracellular envelope proteins A56/K2 were recently shown to bind to the A16/G9 subcomplex to suppress virus-induced fusion from within, our results also highlight an evolutionary convergence in which vaccinia viral fusion suppressor proteins regulate membrane fusion by targeting the A16 and G9 components of the viral EFC complex. Finally, we provide evidence that acid (pH 4.7) treatment induced A26 protein and A26-A27 protein complexes of 70 kDa and 90 kDa to dissociate from mature virions, suggesting that the structure of A26 protein is acid sensitive.
Figures



Similar articles
-
Membrane fusion during poxvirus entry.Semin Cell Dev Biol. 2016 Dec;60:89-96. doi: 10.1016/j.semcdb.2016.07.015. Epub 2016 Jul 14. Semin Cell Dev Biol. 2016. PMID: 27423915 Free PMC article. Review.
-
Vaccinia virus A25 and A26 proteins are fusion suppressors for mature virions and determine strain-specific virus entry pathways into HeLa, CHO-K1, and L cells.J Virol. 2010 Sep;84(17):8422-32. doi: 10.1128/JVI.00599-10. Epub 2010 Jun 10. J Virol. 2010. PMID: 20538855 Free PMC article.
-
Mutations Near the N Terminus of Vaccinia Virus G9 Protein Overcome Restrictions on Cell Entry and Syncytium Formation Imposed by the A56/K2 Fusion Regulatory Complex.J Virol. 2020 May 4;94(10):e00077-20. doi: 10.1128/JVI.00077-20. Print 2020 May 4. J Virol. 2020. PMID: 32132239 Free PMC article.
-
Experimental Evolution To Isolate Vaccinia Virus Adaptive G9 Mutants That Overcome Membrane Fusion Inhibition via the Vaccinia Virus A56/K2 Protein Complex.J Virol. 2020 May 4;94(10):e00093-20. doi: 10.1128/JVI.00093-20. Print 2020 May 4. J Virol. 2020. PMID: 32132237 Free PMC article.
-
From crescent to mature virion: vaccinia virus assembly and maturation.Viruses. 2014 Oct 7;6(10):3787-808. doi: 10.3390/v6103787. Viruses. 2014. PMID: 25296112 Free PMC article. Review.
Cited by
-
Structural and functional analyses of viral H2 protein of the vaccinia virus entry fusion complex.J Virol. 2023 Dec 21;97(12):e0134323. doi: 10.1128/jvi.01343-23. Epub 2023 Nov 17. J Virol. 2023. PMID: 37975688 Free PMC article.
-
Which Proteins? The Challenge of Identifying the Protective Antigens for Next-Generation Capripoxvirus Vaccines.Vaccines (Basel). 2025 Feb 22;13(3):219. doi: 10.3390/vaccines13030219. Vaccines (Basel). 2025. PMID: 40266091 Free PMC article. Review.
-
Vaccinia viral protein A27 is anchored to the viral membrane via a cooperative interaction with viral membrane protein A17.J Biol Chem. 2014 Mar 7;289(10):6639-6655. doi: 10.1074/jbc.M114.547372. Epub 2014 Jan 22. J Biol Chem. 2014. PMID: 24451374 Free PMC article.
-
Membrane fusion during poxvirus entry.Semin Cell Dev Biol. 2016 Dec;60:89-96. doi: 10.1016/j.semcdb.2016.07.015. Epub 2016 Jul 14. Semin Cell Dev Biol. 2016. PMID: 27423915 Free PMC article. Review.
-
Widespread Distribution and Evolution of Poxviral Entry-Fusion Complex Proteins in Giant Viruses.Microbiol Spectr. 2023 Mar 13;11(2):e0494422. doi: 10.1128/spectrum.04944-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36912656 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

