GARP regulates the bioavailability and activation of TGFβ
- PMID: 22278742
- PMCID: PMC3302739
- DOI: 10.1091/mbc.E11-12-1018
GARP regulates the bioavailability and activation of TGFβ
Abstract
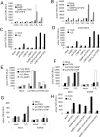
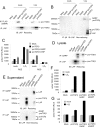
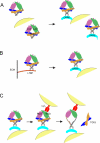
Glycoprotein-A repetitions predominant protein (GARP) associates with latent transforming growth factor-β (proTGFβ) on the surface of T regulatory cells and platelets; however, whether GARP functions in latent TGFβ activation and the structural basis of coassociation remain unknown. We find that Cys-192 and Cys-331 of GARP disulfide link to the TGFβ1 prodomain and that GARP with C192A and C331A mutations can also noncovalently associate with proTGFβ1. Noncovalent association is sufficiently strong for GARP to outcompete latent TGFβ-binding protein for binding to proTGFβ1. Association between GARP and proTGFβ1 prevents the secretion of TGFβ1. Integrin α(V)β(6) and to a lesser extent α(V)β(8) are able to activate TGFβ from the GARP-proTGFβ1 complex. Activation requires the RGD motif of latent TGFβ, disulfide linkage between GARP and latent TGFβ, and membrane association of GARP. Our results show that GARP is a latent TGFβ-binding protein that functions in regulating the bioavailability and activation of TGFβ.
Figures






Similar articles
-
How Soluble GARP Enhances TGFβ Activation.PLoS One. 2016 Apr 7;11(4):e0153290. doi: 10.1371/journal.pone.0153290. eCollection 2016. PLoS One. 2016. PMID: 27054568 Free PMC article.
-
Hepatic Stellate Cells Inhibit T Cells through Active TGF-β1 from a Cell Surface-Bound Latent TGF-β1/GARP Complex.J Immunol. 2015 Sep 15;195(6):2648-56. doi: 10.4049/jimmunol.1500139. Epub 2015 Aug 5. J Immunol. 2015. PMID: 26246140 Free PMC article.
-
Lysosomal-associated Transmembrane Protein 4B (LAPTM4B) Decreases Transforming Growth Factor β1 (TGF-β1) Production in Human Regulatory T Cells.J Biol Chem. 2015 Aug 14;290(33):20105-16. doi: 10.1074/jbc.M115.655340. Epub 2015 Jun 30. J Biol Chem. 2015. PMID: 26126825 Free PMC article.
-
GARP: a surface molecule of regulatory T cells that is involved in the regulatory function and TGF-β releasing.Oncotarget. 2016 Jul 5;7(27):42826-42836. doi: 10.18632/oncotarget.8753. Oncotarget. 2016. PMID: 27095576 Free PMC article. Review.
-
Role of GARP in the activation of latent TGF-β1.Mol Biosyst. 2017 Sep 26;13(10):1925-1935. doi: 10.1039/c7mb00251c. Mol Biosyst. 2017. PMID: 28795730 Review.
Cited by
-
Predictive biomarkers of rapidly developing insulin deficiency in children with type 1 diabetes.BMJ Open Diabetes Res Care. 2024 Feb 27;12(1):e003924. doi: 10.1136/bmjdrc-2023-003924. BMJ Open Diabetes Res Care. 2024. PMID: 38413173 Free PMC article.
-
Therapeutic Approaches for Treating Pulmonary Arterial Hypertension by Correcting Imbalanced TGF-β Superfamily Signaling.Front Med (Lausanne). 2022 Jan 24;8:814222. doi: 10.3389/fmed.2021.814222. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35141256 Free PMC article. Review.
-
Thrombin contributes to cancer immune evasion via proteolysis of platelet-bound GARP to activate LTGF-β.Sci Transl Med. 2020 Jan 8;12(525):eaay4860. doi: 10.1126/scitranslmed.aay4860. Sci Transl Med. 2020. PMID: 31915300 Free PMC article.
-
Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection.Cold Spring Harb Perspect Biol. 2017 Jun 1;9(6):a022236. doi: 10.1101/cshperspect.a022236. Cold Spring Harb Perspect Biol. 2017. PMID: 28108486 Free PMC article. Review.
-
Immunoregulation by members of the TGFβ superfamily.Nat Rev Immunol. 2016 Nov 25;16(12):723-740. doi: 10.1038/nri.2016.112. Nat Rev Immunol. 2016. PMID: 27885276 Review.
References
-
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. - PubMed
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci. 2003;116:217–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

