Multiple domains of human CLASP contribute to microtubule dynamics and organization in vitro and in Xenopus egg extracts
- PMID: 22278908
- PMCID: PMC3315288
- DOI: 10.1002/cm.21005
Multiple domains of human CLASP contribute to microtubule dynamics and organization in vitro and in Xenopus egg extracts
Abstract
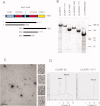
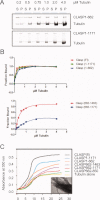
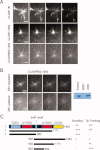
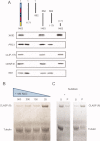
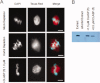
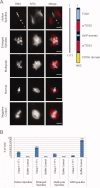
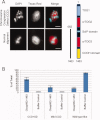
Cytoplasmic linker associated proteins (CLASPs) comprise a class of microtubule (MT) plus end-binding proteins (+TIPs) that contribute to the dynamics and organization of MTs during many cellular processes, among them mitosis. Human CLASP proteins contain multiple MT-binding domains, including tumor over-expressed gene (TOG) domains, and a Ser-x-Ile-Pro (SxIP) motif known to target some +TIPs though interaction with end-binding protein 1 (EB1). However, how individual domains contribute to CLASP function is poorly understood. We generated full-length recombinant human CLASP1 and a series of truncation mutants and found that two N-terminal TOG domains make the strongest contribution to MT polymerization and bundling, but also identified a third TOG domain that further contributes to CLASP activity. Plus end tracking by CLASP requires the SxIP motif and interaction with EB1. The C-terminal coiled-coil domain mediates dimerization and association with many other factors, including the kinetochore motor centromere protein E (CENP-E), and the chromokinesin Xkid. Only the full-length protein was able to rescue spindle assembly in Xenopus egg extracts depleted of endogenous CLASP. Deletion of the C-terminal domain caused aberrant MT polymerization and dramatic spindle phenotypes, even with small amounts of added protein, indicating that proper localization of CLASP activity is essential to control MT polymerization during mitosis.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
Similar articles
-
The role of TOG domains in microtubule plus end dynamics.Biochem Soc Trans. 2009 Oct;37(Pt 5):1002-6. doi: 10.1042/BST0371002. Biochem Soc Trans. 2009. PMID: 19754440 Review.
-
Microtubule plus-end tracking of end-binding protein 1 (EB1) is regulated by CDK5 regulatory subunit-associated protein 2.J Biol Chem. 2017 May 5;292(18):7675-7687. doi: 10.1074/jbc.M116.759746. Epub 2017 Mar 20. J Biol Chem. 2017. PMID: 28320860 Free PMC article.
-
CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex.J Cell Biol. 2005 Jan 3;168(1):141-53. doi: 10.1083/jcb.200405094. J Cell Biol. 2005. PMID: 15631994 Free PMC article.
-
Drosophila melanogaster mini spindles TOG3 utilizes unique structural elements to promote domain stability and maintain a TOG1- and TOG2-like tubulin-binding surface.J Biol Chem. 2015 Apr 17;290(16):10149-62. doi: 10.1074/jbc.M114.633826. Epub 2015 Feb 26. J Biol Chem. 2015. PMID: 25720490 Free PMC article.
-
Regulation of microtubule dynamics by TOG-domain proteins XMAP215/Dis1 and CLASP.Trends Cell Biol. 2011 Oct;21(10):604-14. doi: 10.1016/j.tcb.2011.06.007. Epub 2011 Jul 23. Trends Cell Biol. 2011. PMID: 21782439 Free PMC article. Review.
Cited by
-
Phosphorylation Dynamics Dominate the Regulated Proteome during Early Xenopus Development.Sci Rep. 2017 Nov 15;7(1):15647. doi: 10.1038/s41598-017-15936-y. Sci Rep. 2017. PMID: 29142207 Free PMC article.
-
Synergistic stabilization of microtubules by BUB-1, HCP-1, and CLS-2 controls microtubule pausing and meiotic spindle assembly.Elife. 2023 Feb 17;12:e82579. doi: 10.7554/eLife.82579. Elife. 2023. PMID: 36799894 Free PMC article.
-
Crescerin uses a TOG domain array to regulate microtubules in the primary cilium.Mol Biol Cell. 2015 Nov 15;26(23):4248-64. doi: 10.1091/mbc.E15-08-0603. Epub 2015 Sep 16. Mol Biol Cell. 2015. PMID: 26378256 Free PMC article.
-
CLASPs are required for proper microtubule localization of end-binding proteins.Dev Cell. 2014 Aug 11;30(3):343-52. doi: 10.1016/j.devcel.2014.06.026. Dev Cell. 2014. PMID: 25117684 Free PMC article.
-
CLASP-mediated competitive binding in protein condensates directs microtubule growth.Nat Commun. 2024 Aug 2;15(1):6509. doi: 10.1038/s41467-024-50863-3. Nat Commun. 2024. PMID: 39095354 Free PMC article.
References
-
- Al-Bassam J, Larsen NA, Hyman AA, Harrison SC. Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure. 2007;15:355–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

