Dusky-like functions as a Rab11 effector for the deposition of cuticle during Drosophila bristle development
- PMID: 22278919
- PMCID: PMC3274354
- DOI: 10.1242/dev.074252
Dusky-like functions as a Rab11 effector for the deposition of cuticle during Drosophila bristle development
Abstract
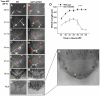
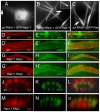
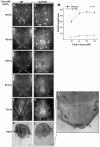
The morphogenesis of Drosophila sensory bristles is dependent on the function of their actin and microtubule cytoskeleton. Actin filaments are important for bristle shape and elongation, while microtubules are thought to mediate protein and membrane trafficking to promote growth. We have identified an essential role for the bristle cuticle in the maintenance of bristle structure and shape at late stages of bristle development. We show that the small GTPase Rab11 mediates the organized deposition of chitin, a major cuticle component in bristles, and disrupting Rab11 function leads to phenotypes that result from bristle collapse rather than a failure to elongate. We further establish that Rab11 is required for the plasma membrane localization of the ZP domain-containing Dusky-like (Dyl) protein and that Dyl is also required for cuticle formation in bristles. Our data argue that Dyl functions as a Rab11 effector for mediating the attachment of the bristle cell membrane to chitin to establish a stable cuticle. Our studies also implicate the exocyst as a Rab11 effector in this process and that Rab11 trafficking along the bristle shaft is mediated by microtubules.
Figures




References
-
- Abdu U., Bar D., Schupbach T. S. (2006). spn-F encodes a novel protein that affects oocyte patterning and bristle morphology in Drosophila. Development 133, 1477–1484 - PubMed
-
- Band A. M., Ali H., Vartiainen M. K., Welti S., Lappalainen P., Olkkonen V. M., Kuismanen E. (2002). Endogenous plasma membrane t-SNARE syntaxin 4 is present in rab11 positive endosomal membranes and associates with cortical actin cytoskeleton. FEBS Lett. 531, 513–519 - PubMed
-
- Bogard N., Lan L., Xu J., Cohen R. S. (2007). Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development 134, 3413–3418 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

