MASTR directs MyoD-dependent satellite cell differentiation during skeletal muscle regeneration
- PMID: 22279050
- PMCID: PMC3273842
- DOI: 10.1101/gad.179663.111
MASTR directs MyoD-dependent satellite cell differentiation during skeletal muscle regeneration
Abstract
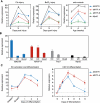
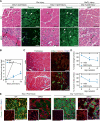
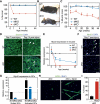
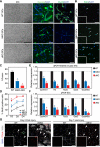
In response to skeletal muscle injury, satellite cells, which function as a myogenic stem cell population, become activated, expand through proliferation, and ultimately fuse with each other and with damaged myofibers to promote muscle regeneration. Here, we show that members of the Myocardin family of transcriptional coactivators, MASTR and MRTF-A, are up-regulated in satellite cells in response to skeletal muscle injury and muscular dystrophy. Global and satellite cell-specific deletion of MASTR in mice impairs skeletal muscle regeneration. This impairment is substantially greater when MRTF-A is also deleted and is due to aberrant differentiation and excessive proliferation of satellite cells. These abnormalities mimic those associated with genetic deletion of MyoD, a master regulator of myogenesis, which is down-regulated in the absence of MASTR and MRTF-A. Consistent with an essential role of MASTR in transcriptional regulation of MyoD expression, MASTR activates a muscle-specific postnatal MyoD enhancer through associations with MEF2 and members of the Myocardin family. Our results provide new insights into the genetic circuitry of muscle regeneration and identify MASTR as a central regulator of this process.
Figures
Similar articles
-
Six1 regulates MyoD expression in adult muscle progenitor cells.PLoS One. 2013 Jun 28;8(6):e67762. doi: 10.1371/journal.pone.0067762. Print 2013. PLoS One. 2013. PMID: 23840772 Free PMC article.
-
Identification of a new hybrid serum response factor and myocyte enhancer factor 2-binding element in MyoD enhancer required for MyoD expression during myogenesis.Mol Biol Cell. 2007 Jun;18(6):1992-2001. doi: 10.1091/mbc.e06-09-0867. Epub 2007 Mar 21. Mol Biol Cell. 2007. PMID: 17377068 Free PMC article.
-
Loss of MyoD and Myf5 in Skeletal Muscle Stem Cells Results in Altered Myogenic Programming and Failed Regeneration.Stem Cell Reports. 2018 Mar 13;10(3):956-969. doi: 10.1016/j.stemcr.2018.01.027. Epub 2018 Mar 1. Stem Cell Reports. 2018. PMID: 29478898 Free PMC article.
-
Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis.Semin Cell Dev Biol. 2017 Dec;72:19-32. doi: 10.1016/j.semcdb.2017.11.011. Epub 2017 Nov 15. Semin Cell Dev Biol. 2017. PMID: 29127046 Review.
-
Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways.Cell Mol Life Sci. 2013 Nov;70(21):4117-30. doi: 10.1007/s00018-013-1330-4. Epub 2013 Apr 4. Cell Mol Life Sci. 2013. PMID: 23552962 Free PMC article. Review.
Cited by
-
Mef2d acts upstream of muscle identity genes and couples lateral myogenesis to dermomyotome formation in Xenopus laevis.PLoS One. 2012;7(12):e52359. doi: 10.1371/journal.pone.0052359. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300648 Free PMC article.
-
Requirement of MEF2A, C, and D for skeletal muscle regeneration.Proc Natl Acad Sci U S A. 2014 Mar 18;111(11):4109-14. doi: 10.1073/pnas.1401732111. Epub 2014 Mar 3. Proc Natl Acad Sci U S A. 2014. PMID: 24591619 Free PMC article.
-
PAX7, a Key for Myogenesis Modulation in Muscular Dystrophies through Multiple Signaling Pathways: A Systematic Review.Int J Mol Sci. 2023 Aug 22;24(17):13051. doi: 10.3390/ijms241713051. Int J Mol Sci. 2023. PMID: 37685856 Free PMC article.
-
Post-transcriptional regulation of MRTF-A by miRNAs during myogenic differentiation of myoblasts.Nucleic Acids Res. 2020 Sep 18;48(16):8927-8942. doi: 10.1093/nar/gkaa596. Nucleic Acids Res. 2020. PMID: 32692361 Free PMC article.
-
The MicroRNA-92a/Sp1/MyoD Axis Regulates Hypoxic Stimulation of Myogenic Lineage Differentiation in Mouse Embryonic Stem Cells.Mol Ther. 2020 Jan 8;28(1):142-156. doi: 10.1016/j.ymthe.2019.08.014. Epub 2019 Sep 3. Mol Ther. 2020. PMID: 31606324 Free PMC article.
References
-
- Asakura A, Lyons GE, Tapscott SJ 1995. The regulation of MyoD gene expression: Conserved elements mediate expression in embryonic axial muscle. Dev Biol 171: 386–398 - PubMed
-
- Banks GB, Chamberlain JS 2008. The value of mammalian models for duchenne muscular dystrophy in developing therapeutic strategies. Curr Top Dev Biol 84: 431–453 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
