A transgenic model of central nervous system autoimmunity mediated by CD4+ and CD8+ T and B cells
- PMID: 22279107
- PMCID: PMC3288950
- DOI: 10.4049/jimmunol.1102186
A transgenic model of central nervous system autoimmunity mediated by CD4+ and CD8+ T and B cells
Abstract
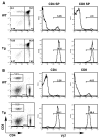
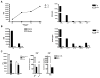
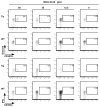
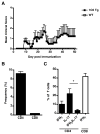
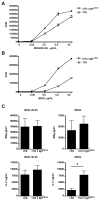
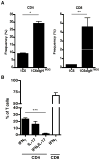
Experimental autoimmune encephalomyelitis (EAE) is a widely used model of multiple sclerosis. In NOD mice, EAE develops as a relapsing-remitting disease that transitions to a chronic progressive disease, making the NOD model the only mouse model that recapitulates the full clinical disease course observed in most multiple sclerosis patients. We have generated a TCR transgenic mouse that expresses the α- and β-chains of a myelin oligodendrocyte glycoprotein (MOG) 35-55-reactive TCR (1C6) on the NOD background. 1C6 TCR transgenic mice spontaneously generate both CD4(+) and CD8(+) T cells that recognize MOG and produce proinflammatory cytokines, allowing for the first time to our knowledge the simultaneous examination of myelin-reactive CD4(+) and CD8(+) T cells in the same host. 1C6 CD8(+) T cells alone can induce optic neuritis and mild EAE with delayed onset; however, 1C6 CD4(+) T cells alone induce severe EAE and predominate in driving disease when both cell types are present. When 1C6 mice are crossed with mice bearing an IgH specific for MOG, the mice develop spontaneous EAE with high incidence, but surprisingly the disease pattern does not resemble the neuromyelitis optica-like disease observed in mice bearing CD4(+) T cells and B cells reactive to MOG on the C57BL/6 background. Collectively, our data show that although myelin-reactive CD8(+) T cells contribute to disease, disease is primarily driven by myelin-reactive CD4(+) T cells and that the coexistence of myelin-reactive T and B cells does not necessarily result in a distinct pathological phenotype.
Figures

References
-
- Encinas JA, Wicker LS, Peterson LB, Mukasa A, Teuscher C, Sobel R, Weiner HL, Seidman CE, Seidman JG, Kuchroo VK. QTL influencing autoimmune diabetes and encephalomyelitis map to a 0.15-cM region containing IL2. Nat Genet. 1999;21:158–160. - PubMed
-
- Vyse TJ, Todd JA. Genetic analysis of autoimmune disease. Cell. 1996;85:311–318. - PubMed
-
- Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein -specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. - PubMed
-
- Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low Avidity Recognition of Self-Antigen by T Cells Permits Escape from Central Tolerance. Immunity. 1995;3:407–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

