Serotonin modulates multiple calcium current subtypes in commissural interneurons of the neonatal mouse
- PMID: 22279189
- PMCID: PMC3331599
- DOI: 10.1152/jn.00768.2011
Serotonin modulates multiple calcium current subtypes in commissural interneurons of the neonatal mouse
Abstract
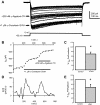
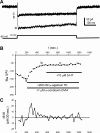
Calcium currents are critical to the intrinsic properties of neurons and the networks that contain them. These currents make attractive targets for neuromodulation. Here, we examine the serotonergic modulation of specific calcium current subtypes in neonatal (P0-5) intersegmental commissural interneurons (CINs), members of the hindlimb locomotor central pattern generator in the mouse spinal cord. Previous work in our lab showed that serotonin (5-HT) excited CINs in part by reducing a calcium current and thus indirectly reducing the calcium-activated potassium current (Diaz-Rios et al. 2007). We have determined which calcium currents are targets of serotonin modulation. Utilizing whole cell voltage clamp and toxins to specific calcium current subtypes, we found that N- and P/Q-type currents comprise over 60% of the overall calcium current. Blockade of each of these subtypes alone with either ω-conotoxin GVIA or ω-agatoxin TK was unable to occlude 5-HT's reduction of the calcium current. However, coapplication of both blockers together fully occluded 5-HT's reduction of the calcium current. Thus, 5-HT decreases both N- and P/Q-type calcium current to excite neonatal CINs.
Figures




References
-
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 314: 615–620, 2006 - PubMed
-
- Buschges A, Wikstrom MA, Grillner S, El Manira A. Roles of high-voltage-activated calcium channel subtypes in a vertebrate spinal locomotor network. J Neurophysiol 84: 2758–2766, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

