Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits
- PMID: 22279209
- PMCID: PMC3567460
- DOI: 10.1523/JNEUROSCI.2277-11.2012
Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits
Abstract
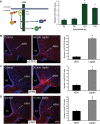
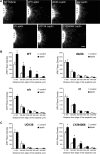
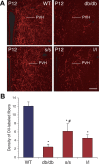
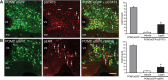
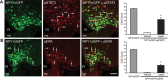
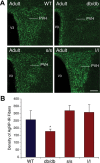
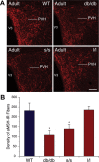
Circulating hormones influence multiple aspects of hypothalamic development and play a role in directing formation of neural circuits. Leptin is secreted by adipocytes and functions as a key developmental signal that promotes axon outgrowth from the arcuate nucleus (ARH) during a discrete developmental critical period. To determine the cellular mechanisms by which leptin impacts development of hypothalamic circuits, we examined roles for leptin receptor (LepRb) signals in neonatal mice. LepRb, ERK, and STAT3 signaling were required for leptin-stimulated neurite outgrowth from ARH explants in vitro. Neonatal mice with disrupted LepRb→ERK signaling displayed impaired ARH projections but were able to compensate by adulthood. LepRb→STAT3 signaling also plays a role in early circuit formation and controls the ultimate architecture of POMC, but not AgRP, projections. Thus, the developmental actions of leptin on feeding circuits are dependent on LepRb, and distinct signaling pathways are responsible for directing formation of NPY and POMC projections.
Figures
References
-
- Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. - PubMed
-
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. - PubMed
-
- Bjørbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
