Similar intracellular Ca2+ requirements for inactivation and facilitation of voltage-gated Ca2+ channels in a glutamatergic mammalian nerve terminal
- PMID: 22279211
- PMCID: PMC6796276
- DOI: 10.1523/JNEUROSCI.3838-11.2012
Similar intracellular Ca2+ requirements for inactivation and facilitation of voltage-gated Ca2+ channels in a glutamatergic mammalian nerve terminal
Abstract
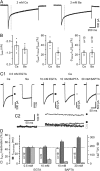
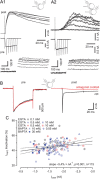
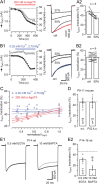
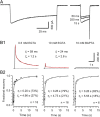
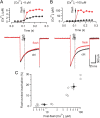
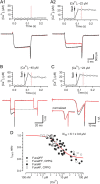
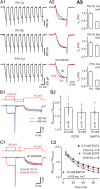
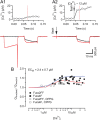
Voltage-gated Ca2+ channels (VGCCs) of the P/Q-type, which are expressed at a majority of mammalian nerve terminals, show two types of Ca2+-dependent feedback regulation-inactivation (CDI) and facilitation (CDF). Because of the nonlinear relationship between Ca2+ influx and transmitter release, CDI and CDF are powerful regulators of synaptic strength. To what extent VGCCs inactivate or facilitate during spike trains depends on the dynamics of free Ca2+ ([Ca2+]i) and the Ca2+ sensitivity of CDI and CDF, which has not been determined in nerve terminals. In this report, we took advantage of the large size of a rat auditory glutamatergic synapse--the calyx of Held--and combined voltage-clamp recordings of presynaptic Ca2+ currents (ICa(V)) with UV-light flash-induced Ca2+ uncaging and presynaptic Ca2+ imaging to study the Ca2+ requirements for CDI and CDF. We find that nearly half of the presynaptic VGCCs inactivate during 100 ms voltage steps and require several seconds to recover. This inactivation is caused neither by depletion of Ca2+ ions from the synaptic cleft nor by metabotropic feedback inhibition, because it is resistant to blockade of metabotropic and ionotropic glutamate receptors. Facilitation of ICa(V) induced by repetitive depolarizations or preconditioning voltage steps decays within tens of milliseconds. Since Ca2+ buffers only weakly affect CDI and CDF, we conclude that the Ca2+ sensors are closely associated with the channel. CDI and CDF can be induced by intracellular photo release of Ca2+ resulting in [Ca2+]i elevations in the low micromolar range, implying a surprisingly high affinity of the Ca2+ sensors.
Figures
Similar articles
-
Neurosteroid pregnenolone sulfate enhances glutamatergic synaptic transmission by facilitating presynaptic calcium currents at the calyx of Held of immature rats.Eur J Neurosci. 2006 Oct;24(7):1955-66. doi: 10.1111/j.1460-9568.2006.05080.x. Epub 2006 Oct 16. Eur J Neurosci. 2006. PMID: 17040476
-
Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice.J Physiol. 2005 Oct 1;568(Pt 1):199-209. doi: 10.1113/jphysiol.2005.089912. Epub 2005 Jul 21. J Physiol. 2005. PMID: 16037093 Free PMC article.
-
Voltage-gated Ca2+ channel subtypes mediating GABAergic transmission in the rat supraoptic nucleus.Eur J Neurosci. 2005 May;21(9):2459-66. doi: 10.1111/j.1460-9568.2005.04097.x. Eur J Neurosci. 2005. PMID: 15932603
-
Diversity of presynaptic calcium channels displaying different synaptic properties.Rev Neurosci. 2012 Feb 29;23(2):179-90. doi: 10.1515/revneuro-2011-0070. Rev Neurosci. 2012. PMID: 22499676 Review.
-
Ca2+-dependent modulation of voltage-gated Ca2+ channels.Biochim Biophys Acta. 2012 Aug;1820(8):1243-52. doi: 10.1016/j.bbagen.2011.12.012. Epub 2011 Dec 24. Biochim Biophys Acta. 2012. PMID: 22223119 Free PMC article. Review.
Cited by
-
Sustaining rapid vesicular release at active zones: potential roles for vesicle tethering.Trends Neurosci. 2013 Mar;36(3):185-94. doi: 10.1016/j.tins.2012.10.001. Epub 2012 Nov 17. Trends Neurosci. 2013. PMID: 23164531 Free PMC article. Review.
-
Synaptic gain-of-function effects of mutant Cav2.1 channels in a mouse model of familial hemiplegic migraine are due to increased basal [Ca2+]i.J Neurosci. 2014 May 21;34(21):7047-58. doi: 10.1523/JNEUROSCI.2526-13.2014. J Neurosci. 2014. PMID: 24849341 Free PMC article.
-
Calretinin regulates Ca2+-dependent inactivation and facilitation of Ca(v)2.1 Ca2+ channels through a direct interaction with the α12.1 subunit.J Biol Chem. 2012 Nov 16;287(47):39766-75. doi: 10.1074/jbc.M112.406363. Epub 2012 Oct 2. J Biol Chem. 2012. PMID: 23033479 Free PMC article.
-
Ca2+ channel to synaptic vesicle distance accounts for the readily releasable pool kinetics at a functionally mature auditory synapse.J Neurosci. 2015 Feb 4;35(5):2083-100. doi: 10.1523/JNEUROSCI.2753-14.2015. J Neurosci. 2015. PMID: 25653365 Free PMC article.
-
Dynamics of volume-averaged intracellular Ca2+ in a rat CNS nerve terminal during single and repetitive voltage-clamp depolarizations.J Physiol. 2017 May 15;595(10):3219-3236. doi: 10.1113/JP272773. Epub 2017 Feb 1. J Physiol. 2017. PMID: 27957749 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
