The α1K276E startle disease mutation reveals multiple intermediate states in the gating of glycine receptors
- PMID: 22279218
- PMCID: PMC6796251
- DOI: 10.1523/JNEUROSCI.4346-11.2012
The α1K276E startle disease mutation reveals multiple intermediate states in the gating of glycine receptors
Abstract
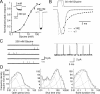
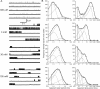
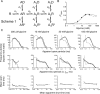
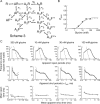
Loss-of-function mutations in human glycine receptors cause hyperekplexia, a rare inherited disease associated with an exaggerated startle response. We have studied a human disease mutation in the M2-M3 loop of the glycine receptor α1 subunit (K276E) using direct fitting of mechanisms to single-channel recordings with the program HJCFIT. Whole-cell recordings from HEK293 cells showed the mutation reduced the receptor glycine sensitivity. In single-channel recordings, rat homomeric α1 K276E receptors were barely active, even at 200 mM glycine. Coexpression of the β subunit partially rescued channel function. Heteromeric mutant channels opened in brief bursts at 300 μM glycine (a concentration that is near-maximal for wild type) and reached a maximum one-channel open probability of about 45% at 100 mm glycine (compared to 96% for wild type). Distributions of apparent open times contained more than one component in high glycine and, therefore, could not be described by mechanisms with only one fully liganded open state. Fits to the data were much better with mechanisms in which opening can also occur from more than one fully liganded intermediate (e.g., "primed" models). Brief pulses of glycine (∼3 ms, 30 mM) applied to mutant channels in outside-out patches activated currents with a slower rise time (1.5 ms) than those of wild-type channels (0.2 ms) and a much faster decay. These features were predicted reasonably well by the mechanisms obtained from fitting single-channel data. Our results show that, by slowing and impairing channel gating, the K276E mutation facilitates the detection of closed reaction intermediates in the activation pathway of glycine channels.
Figures




References
-
- Ball FG, Milne RK, Yeo GF. Marked continuous-time Markov chain modelling of burst behaviour for single ion channels. J Appl Math Decis Sci. 2007;2007 doi: 10.1155/2007/48138. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
