A block in lineage differentiation of immortal human mammary stem / progenitor cells by ectopically-expressed oncogenes
- PMID: 22279424
- PMCID: PMC3263028
- DOI: 10.4103/1477-3163.91415
A block in lineage differentiation of immortal human mammary stem / progenitor cells by ectopically-expressed oncogenes
Abstract
Introduction: Emerging evidence suggests a direct role of cancer stem cells (CSCs) in the development of breast cancer. In vitro cellular models that recapitulate properties of CSCs are therefore highly desirable. We have previously shown that normal human mammary epithelial cells (hMECs) immortalized with human telomerase reverse transcriptase (hTERT) possess properties of mammary stem / progenitor cells.
Materials and methods: In the present study, we used this cell system to test the idea that other known hMEC-immortalizing oncogenes (RhoA, HPVE6, HPVE7, p53 mutant, and treatment with γ-radiation), share with hTERT, the ability to maintain mammary stem / progenitor cells.
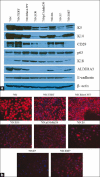
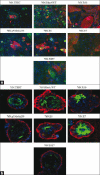
Results: The results presented here demonstrate that similar to hMECs immortalized with hTERT, all hMEC cell lines immortalized using various oncogenic strategies express stem / progenitor cell markers. Furthermore, analyses using 2D and 3D culture assays demonstrate that all the immortal cell lines retain their ability to self-renew and to differentiate along the luminal lineage. Remarkably, the stem / progenitor cell lines generated using various oncogenic strategies exhibit a block in differentiation along the myoepithelial lineage, a trait that is retained on hTERT-immortalized stem / progenitors. The inability to differentiate along the myoepithelial lineage could be induced by ectopic mutant p53 expression in hTERT-immortalized hMEC.
Conclusions: Our studies demonstrate that stem / progenitor cell characteristics of hMECs are maintained upon immortalization by using various cancer-relevant oncogenic strategies. Oncogene-immortalized hMECs show a block in their ability to differentiate along the myoepithelial lineage. Abrogation of the myoepithelial differentiation potential by a number of distinct oncogenic insults suggests a potential explanation for the predominance of luminal and rarity of myoepithelial breast cancers.
Keywords: Immortalization; luminal differentiation; myoepithelial differentiation; self-renewal; stem cell.
Figures

Similar articles
-
Derivation of myoepithelial progenitor cells from bipotent mammary stem/progenitor cells.PLoS One. 2012;7(4):e35338. doi: 10.1371/journal.pone.0035338. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514728 Free PMC article.
-
Telomerase-immortalized human mammary stem/progenitor cells with ability to self-renew and differentiate.Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14146-51. doi: 10.1073/pnas.1009030107. Epub 2010 Jul 26. Proc Natl Acad Sci U S A. 2010. PMID: 20660721 Free PMC article.
-
Distinct effects of EGFR ligands on human mammary epithelial cell differentiation.PLoS One. 2013 Oct 4;8(10):e75907. doi: 10.1371/journal.pone.0075907. eCollection 2013. PLoS One. 2013. PMID: 24124521 Free PMC article.
-
Mammary epithelial cell transformation: insights from cell culture and mouse models.Breast Cancer Res. 2005;7(4):171-9. doi: 10.1186/bcr1275. Epub 2005 Jun 3. Breast Cancer Res. 2005. PMID: 15987472 Free PMC article. Review.
-
Epithelial progenitors in the normal human mammary gland.J Mammary Gland Biol Neoplasia. 2005 Jan;10(1):49-59. doi: 10.1007/s10911-005-2540-7. J Mammary Gland Biol Neoplasia. 2005. PMID: 15886886 Review.
Cited by
-
Derivation of myoepithelial progenitor cells from bipotent mammary stem/progenitor cells.PLoS One. 2012;7(4):e35338. doi: 10.1371/journal.pone.0035338. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514728 Free PMC article.
-
Mutant PIK3CA Induces EMT in a Cell Type Specific Manner.PLoS One. 2016 Dec 12;11(12):e0167064. doi: 10.1371/journal.pone.0167064. eCollection 2016. PLoS One. 2016. PMID: 27941987 Free PMC article.
-
Cell type of origin as well as genetic alterations contribute to breast cancer phenotypes.Oncotarget. 2015 Apr 20;6(11):9018-30. doi: 10.18632/oncotarget.3379. Oncotarget. 2015. PMID: 25940703 Free PMC article.
-
Elevated expression of CST1 promotes breast cancer progression and predicts a poor prognosis.J Mol Med (Berl). 2017 Aug;95(8):873-886. doi: 10.1007/s00109-017-1537-1. Epub 2017 May 18. J Mol Med (Berl). 2017. PMID: 28523467 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Morrow PK, Hortobagyi GN. Management of breast cancer in the genome era. Annu Rev Med. 2009;60:153–65. - PubMed
-
- Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol. 2009;27:44–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

