Largazole and its derivatives selectively inhibit ubiquitin activating enzyme (e1)
- PMID: 22279528
- PMCID: PMC3261141
- DOI: 10.1371/journal.pone.0029208
Largazole and its derivatives selectively inhibit ubiquitin activating enzyme (e1)
Abstract
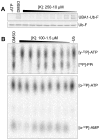
Protein ubiquitination plays an important role in the regulation of almost every aspect of eukaryotic cellular function; therefore, its destabilization is often observed in most human diseases and cancers. Consequently, developing inhibitors of the ubiquitination system for the treatment of cancer has been a recent area of interest. Currently, only a few classes of compounds have been discovered to inhibit the ubiquitin-activating enzyme (E1) and only one class is relatively selective in E1 inhibition in cells. We now report that Largazole and its ester and ketone analogs selectively inhibit ubiquitin conjugation to p27(Kip1) and TRF1 in vitro. The inhibitory activity of these small molecules on ubiquitin conjugation has been traced to their inhibition of the ubiquitin E1 enzyme. To further dissect the mechanism of E1 inhibition, we analyzed the effects of these inhibitors on each of the two steps of E1 activation. We show that Largazole and its derivatives specifically inhibit the adenylation step of the E1 reaction while having no effect on thioester bond formation between ubiquitin and E1. E1 inhibition appears to be specific to human E1 as Largazole ketone fails to inhibit the activation of Uba1p, a homolog of E1 in Schizosaccharomyces pombe. Moreover, Largazole analogs do not significantly inhibit SUMO E1. Thus, Largazole and select analogs are a novel class of ubiquitin E1 inhibitors and valuable tools for studying ubiquitination in vitro. This class of compounds could be further developed and potentially be a useful tool in cells.
Conflict of interest statement
Figures



Similar articles
-
Identification and mechanistic studies of a novel ubiquitin E1 inhibitor.J Biomol Screen. 2012 Apr;17(4):421-34. doi: 10.1177/1087057111433843. Epub 2012 Jan 24. J Biomol Screen. 2012. PMID: 22274912 Free PMC article.
-
Domain alternation and active site remodeling are conserved structural features of ubiquitin E1.J Biol Chem. 2017 Jul 21;292(29):12089-12099. doi: 10.1074/jbc.M117.787622. Epub 2017 Jun 1. J Biol Chem. 2017. PMID: 28572513 Free PMC article.
-
Design, synthesis, and biological evaluation of novel ubiquitin-activating enzyme inhibitors.Bioorg Med Chem Lett. 2018 Sep 1;28(16):2723-2727. doi: 10.1016/j.bmcl.2018.03.004. Epub 2018 Mar 3. Bioorg Med Chem Lett. 2018. PMID: 29548576
-
Synthetic routes and biological evaluation of largazole and its analogues as potent histone deacetylase inhibitors.Molecules. 2011 Jun 7;16(6):4681-94. doi: 10.3390/molecules16064681. Molecules. 2011. PMID: 21654576 Free PMC article. Review.
-
Largazole Analogues as Histone Deacetylase Inhibitors and Anticancer Agents: An Overview of Structure-Activity Relationships.ChemMedChem. 2017 Dec 7;12(23):1917-1926. doi: 10.1002/cmdc.201700563. Epub 2017 Nov 17. ChemMedChem. 2017. PMID: 29117473 Review.
Cited by
-
Marine Cyanobacteria as Sources of Lead Anticancer Compounds: A Review of Families of Metabolites with Cytotoxic, Antiproliferative, and Antineoplastic Effects.Molecules. 2022 Jul 27;27(15):4814. doi: 10.3390/molecules27154814. Molecules. 2022. PMID: 35956762 Free PMC article. Review.
-
Natural Cyclopeptides as Anticancer Agents in the Last 20 Years.Int J Mol Sci. 2021 Apr 12;22(8):3973. doi: 10.3390/ijms22083973. Int J Mol Sci. 2021. PMID: 33921480 Free PMC article. Review.
-
Marine Cyanobacteria: A Source of Lead Compounds and their Clinically-Relevant Molecular Targets.Molecules. 2020 May 8;25(9):2197. doi: 10.3390/molecules25092197. Molecules. 2020. PMID: 32397127 Free PMC article. Review.
-
Repurposing old drugs as new inhibitors of the ubiquitin-proteasome pathway for cancer treatment.Semin Cancer Biol. 2021 Jan;68:105-122. doi: 10.1016/j.semcancer.2019.12.013. Epub 2019 Dec 26. Semin Cancer Biol. 2021. PMID: 31883910 Free PMC article. Review.
-
Adverse Reaction to COVID-19 mRNA Vaccination in a Patient With VEXAS Syndrome.Cureus. 2022 Mar 24;14(3):e23456. doi: 10.7759/cureus.23456. eCollection 2022 Mar. Cureus. 2022. PMID: 35481304 Free PMC article.
References
-
- Hershko A, Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. - PubMed
-
- Haas AL, Warms JV, Hershko A, Rose IA. Ubiquitin-activating enzyme. Mechanism and role in protein-ubiquitin conjugation. J Biol Chem. 1982;257:2543–2548. - PubMed
-
- Hershko A, Heller H. Occurrence of a polyubiquitin structure in ubiquitin-protein conjugates. Biochem Biophys Res Commun. 1985;128:1079–1086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

