A new model for raf kinase inhibitory protein induced chemotherapeutic resistance
- PMID: 22279539
- PMCID: PMC3261143
- DOI: 10.1371/journal.pone.0029532
A new model for raf kinase inhibitory protein induced chemotherapeutic resistance
Abstract
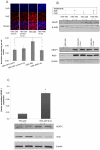
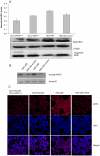
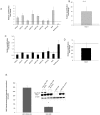
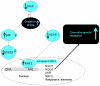
Therapeutic resistance remains the most challenging aspect of treating cancer. Raf kinase inhibitory protein (RKIP) emerged as a molecule capable of sensitizing cancerous cells to radio- and chemotherapy. Moreover, this small evolutionary conserved molecule, endows significant resistance to cancer therapy when its expression is reduced or lost. RKIP has been shown to inhibit the Raf-MEK-ERK, NFκB, GRK and activate the GSK3β signaling pathways. Inhibition of Raf-MEK-ERK and NFκB remains the most prominent pathways implicated in the sensitization of cells to therapeutic drugs. Our purpose was to identify a possible link between RKIP-KEAP 1-NRF2 and drug resistance. To that end, RKIP-KEAP 1 association was tested in human colorectal cancer tissues using immunohistochemistry. RKIP miRNA silencing and its inducible overexpression were employed in HEK-293 immortalized cells, HT29 and HCT116 colon cancer cell lines to further investigate our aim. We show that RKIP enhanced Kelch-like ECH-associated protein1 (KEAP 1) stability in colorectal cancer tissues and HT29 CRC cell line. RKIP silencing in immortalized HEK-293 cells (termed HEK-499) correlated significantly with KEAP 1 protein degradation and subsequent NRF2 addiction in these cells. Moreover, RKIP depletion in HEK-499, compared to control cells, bestowed resistance to supra physiological levels of H(2)O(2) and Cisplatin possibly by upregulating NF-E2-related nuclear factor 2 (NRF2) responsive genes. Similarly, we observed a direct correlation between the extent of apoptosis, after treatment with Adriamycin, and the expression levels of RKIP/KEAP 1 in HT29 but not in HCT116 CRC cells. Our data illuminate, for the first time, the NRF2-KEAP 1 pathway as a possible target for personalized therapeutic intervention in RKIP depleted cancers.
Conflict of interest statement
Figures




Similar articles
-
Effect of Raf kinase inhibitor protein expression on malignant biological behavior and progression of colorectal cancer.Oncol Rep. 2015 Oct;34(4):2106-14. doi: 10.3892/or.2015.4157. Epub 2015 Jul 28. Oncol Rep. 2015. PMID: 26238523
-
Pivotal roles of snail inhibition and RKIP induction by the proteasome inhibitor NPI-0052 in tumor cell chemoimmunosensitization.Cancer Res. 2009 Nov 1;69(21):8376-85. doi: 10.1158/0008-5472.CAN-09-1069. Epub 2009 Oct 20. Cancer Res. 2009. PMID: 19843864
-
RKIP promotes cisplatin-induced gastric cancer cell death through NF-κB/Snail pathway.Tumour Biol. 2015 Mar;36(3):1445-53. doi: 10.1007/s13277-014-2496-6. Epub 2014 Dec 30. Tumour Biol. 2015. PMID: 25547433
-
Raf-1 kinase inhibitor protein: structure, function, regulation of cell signaling, and pivotal role in apoptosis.Adv Cancer Res. 2004;91:169-200. doi: 10.1016/S0065-230X(04)91005-6. Adv Cancer Res. 2004. PMID: 15327891 Review.
-
Raf kinase inhibitor protein (RKIP) in cancer.Cancer Metastasis Rev. 2012 Dec;31(3-4):615-20. doi: 10.1007/s10555-012-9365-9. Cancer Metastasis Rev. 2012. PMID: 22684368 Review.
Cited by
-
A Negative Regulatory Role for RKIP in Breast Cancer Immune Response.Cancers (Basel). 2022 Jul 24;14(15):3605. doi: 10.3390/cancers14153605. Cancers (Basel). 2022. PMID: 35892864 Free PMC article.
-
RKIP-mediated chemo-immunosensitization of resistant cancer cells via disruption of the NF-κB/Snail/YY1/RKIP resistance-driver loop.Crit Rev Oncog. 2014;19(6):431-45. doi: 10.1615/critrevoncog.2014011929. Crit Rev Oncog. 2014. PMID: 25597353 Free PMC article. Review.
-
Geographic analysis of RKIP expression and its clinical relevance in colorectal cancer.Br J Cancer. 2013 May 28;108(10):2088-96. doi: 10.1038/bjc.2013.197. Epub 2013 Apr 30. Br J Cancer. 2013. PMID: 23632477 Free PMC article.
-
Raf kinase inhibitor protein mediates myocardial fibrosis under conditions of enhanced myocardial oxidative stress.Basic Res Cardiol. 2018 Sep 6;113(6):42. doi: 10.1007/s00395-018-0700-3. Basic Res Cardiol. 2018. PMID: 30191336 Free PMC article.
-
RKIP Inhibits Local Breast Cancer Invasion by Antagonizing the Transcriptional Activation of MMP13.PLoS One. 2015 Aug 26;10(8):e0134494. doi: 10.1371/journal.pone.0134494. eCollection 2015. PLoS One. 2015. PMID: 26308852 Free PMC article.
References
-
- Al-Mulla F, Hagan S, Behbehani AI, Bitar MS, George SS, et al. Raf kinase inhibitor protein expression in a survival analysis of colorectal cancer patients. J Clin Oncol. 2006;24:5672–5679. - PubMed
-
- Chen Y, Tang CE, Ouyang GL, Ruan L, Li MY, et al. Identification of RKIP as a differentially tyrosine-phosphorylated protein in nasopharyngeal carcinoma and normal nasopharyngeal epithelial tissues by phosphoproteomic approach. Med Oncol. 2009;26:463–470. - PubMed
-
- Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R, et al. Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. Prostate. 2006;66:248–256. - PubMed
-
- Hagan S, Al-Mulla F, Mallon E, Oien K, Ferrier R, et al. Reduction of Raf-1 kinase inhibitor protein expression correlates with breast cancer metastasis. Clin Cancer Res. 2005;11:7392–7397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

