TRAPPC9 mediates the interaction between p150 and COPII vesicles at the target membrane
- PMID: 22279557
- PMCID: PMC3261171
- DOI: 10.1371/journal.pone.0029995
TRAPPC9 mediates the interaction between p150 and COPII vesicles at the target membrane
Abstract
Background: The transport of endoplasmic reticulum (ER)-derived COPII vesicles toward the ER-Golgi intermediate compartment (ERGIC) requires cytoplasmic dynein and is dependent on microtubules. p150(Glued), a subunit of dynactin, has been implicated in the transport of COPII vesicles via its interaction with COPII coat components Sec23 and Sec24. However, whether and how COPII vesicle tether, TRAPP (Transport protein particle), plays a role in the interaction between COPII vesicles and microtubules is currently unknown.
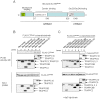
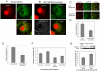
Principle findings: We address the functional relationship between COPII tether TRAPP and dynactin. Overexpressed TRAPP subunits interfered with microtubule architecture by competing p150(Glued) away from the MTOC. TRAPP subunit TRAPPC9 bound directly to p150(Glued) via the same carboxyl terminal domain of p150(Glued) that binds Sec23 and Sec24. TRAPPC9 also inhibited the interaction between p150(Glued) and Sec23/Sec24 both in vitro and in vivo, suggesting that TRAPPC9 serves to uncouple p150(Glued) from the COPII coat, and to relay the vesicle-dynactin interaction at the target membrane.
Conclusions: These findings provide a new perspective on the function of TRAPP as an adaptor between the ERGIC membrane and dynactin. By preserving the connection between dynactin and the tethered and/or fused vesicles, TRAPP allows nascent ERGIC to continue the movement along the microtubules as they mature into the cis-Golgi.
Conflict of interest statement
Figures






References
-
- Roghi C, Allan VJ. Dynamic association of cytoplasmic dynein heavy chain 1a with the Golgi apparatus and intermediate compartment. J Cell Sci. 1999;112:4673–4685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

