The chemotactic defect in wiskott-Aldrich syndrome macrophages is due to the reduced persistence of directional protrusions
- PMID: 22279563
- PMCID: PMC3261183
- DOI: 10.1371/journal.pone.0030033
The chemotactic defect in wiskott-Aldrich syndrome macrophages is due to the reduced persistence of directional protrusions
Abstract
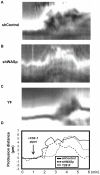
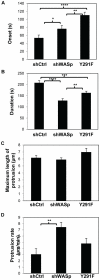
Wiskott-Aldrich syndrome protein (WASp) is an actin nucleation promoting factor that is required for macrophages to directionally migrate towards various chemoattractants. The chemotaxis defect of WASp-deficient cells and its activation by Cdc42 in vivo suggest that WASp plays a role in directional sensing, however, its precise role in macrophage chemotaxis is still unclear. Using shRNA-mediated downregulation of WASp in the murine monocyte/macrophage cell line RAW/LR5 (shWASp), we found that WASp was responsible for the initial wave of actin polymerization in response to global stimulation with CSF-1, which in Dictyostelium discoideum amoebae and carcinoma cells has been correlated with the ability to migrate towards chemoattractants. Real-time monitoring of shWASp cells, as well as WASp⁻/⁻ bone marrow-derived macrophages (BMMs), in response to a CSF-1 gradient revealed that the protrusions from WASp-deficient cells were directional, showing intact directional sensing. However, the protrusions from WASp-deficient cells demonstrated reduced persistence compared to their respective control shRNA and wild-type cells. Further examination showed that tyrosine phosphorylation of WASp was required for both the first wave of actin polymerization following global CSF-1 stimulation and proper directional responses towards CSF-1. Importantly, the PI3K, Rac1 and WAVE2 proteins were incorporated normally in CSF-1 - elicited protrusions in the absence of WASp, suggesting that membrane protrusion driven by the WAVE2 complex signaling is intact. Collectively, these results suggest that WASp and its phosphorylation play critical roles in coordinating the actin cytoskeleton rearrangements necessary for the persistence of protrusions required for directional migration of macrophages towards CSF-1.
Conflict of interest statement
Figures



Similar articles
-
A WAVE2-Abi1 complex mediates CSF-1-induced F-actin-rich membrane protrusions and migration in macrophages.J Cell Sci. 2005 Nov 15;118(Pt 22):5369-79. doi: 10.1242/jcs.02638. J Cell Sci. 2005. PMID: 16280551
-
N-WASP has the ability to compensate for the loss of WASP in macrophage podosome formation and chemotaxis.Exp Cell Res. 2010 Dec 10;316(20):3406-16. doi: 10.1016/j.yexcr.2010.06.011. Epub 2010 Jun 27. Exp Cell Res. 2010. PMID: 20599953 Free PMC article.
-
Membrane targeting of WAVE2 is not sufficient for WAVE2-dependent actin polymerization: a role for IRSp53 in mediating the interaction between Rac and WAVE2.J Cell Sci. 2008 Feb 1;121(Pt 3):379-90. doi: 10.1242/jcs.010272. Epub 2008 Jan 15. J Cell Sci. 2008. PMID: 18198193 Free PMC article.
-
WASP (Wiskott-Aldrich syndrome protein) gene mutations and phenotype.Curr Opin Allergy Clin Immunol. 2003 Dec;3(6):427-36. doi: 10.1097/00130832-200312000-00003. Curr Opin Allergy Clin Immunol. 2003. PMID: 14612666 Review.
-
The Wiskott-Aldrich syndrome: The actin cytoskeleton and immune cell function.Dis Markers. 2010;29(3-4):157-75. doi: 10.3233/DMA-2010-0735. Dis Markers. 2010. PMID: 21178275 Free PMC article. Review.
Cited by
-
Actin cytoskeletal defects in immunodeficiency.Immunol Rev. 2013 Nov;256(1):282-99. doi: 10.1111/imr.12114. Immunol Rev. 2013. PMID: 24117828 Free PMC article. Review.
-
Regulation of the Actin Cytoskeleton via Rho GTPase Signalling in Dictyostelium and Mammalian Cells: A Parallel Slalom.Cells. 2021 Jun 24;10(7):1592. doi: 10.3390/cells10071592. Cells. 2021. PMID: 34202767 Free PMC article. Review.
-
Molecular difference between WASP and N-WASP critical for chemotaxis of T-cells towards SDF-1α.Sci Rep. 2015 Oct 14;5:15031. doi: 10.1038/srep15031. Sci Rep. 2015. PMID: 26463123 Free PMC article.
-
Modelling of human Wiskott-Aldrich syndrome protein mutants in zebrafish larvae using in vivo live imaging.J Cell Sci. 2013 Sep 15;126(Pt 18):4077-84. doi: 10.1242/jcs.128728. Epub 2013 Jul 18. J Cell Sci. 2013. PMID: 23868979 Free PMC article. Review.
-
Targeting the actin nucleation promoting factor WASp provides a therapeutic approach for hematopoietic malignancies.Nat Commun. 2021 Sep 22;12(1):5581. doi: 10.1038/s41467-021-25842-7. Nat Commun. 2021. PMID: 34552085 Free PMC article.
References
-
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Current opinion in cell biology. 2003;15:590–597. - PubMed
-
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. - PubMed
-
- Parent CA, Devreotes PN. A cell's sense of direction. Science. 1999;284:765–770. - PubMed
-
- Calle Y, Burns S, Thrasher AJ, Jones GE. The leukocyte podosome. European journal of cell biology. 2006;85:151–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

