CNS penetration of intrathecal-lumbar idursulfase in the monkey, dog and mouse: implications for neurological outcomes of lysosomal storage disorder
- PMID: 22279584
- PMCID: PMC3261205
- DOI: 10.1371/journal.pone.0030341
CNS penetration of intrathecal-lumbar idursulfase in the monkey, dog and mouse: implications for neurological outcomes of lysosomal storage disorder
Abstract
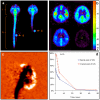
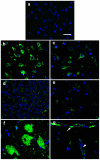
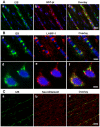
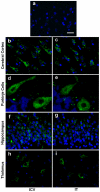
A major challenge for the treatment of many central nervous system (CNS) disorders is the lack of convenient and effective methods for delivering biological agents to the brain. Mucopolysaccharidosis II (Hunter syndrome) is a rare inherited lysosomal storage disorder resulting from a deficiency of iduronate-2-sulfatase (I2S). I2S is a large, highly glycosylated enzyme. Intravenous administration is not likely to be an effective therapy for disease-related neurological outcomes that require enzyme access to the brain cells, in particular neurons and oligodendrocytes. We demonstrate that intracerebroventricular and lumbar intrathecal administration of recombinant I2S in dogs and nonhuman primates resulted in widespread enzyme distribution in the brain parenchyma, including remarkable deposition in the lysosomes of both neurons and oligodendrocytes. Lumbar intrathecal administration also resulted in enzyme delivery to the spinal cord, whereas little enzyme was detected there after intraventricular administration. Mucopolysaccharidosis II model is available in mice. Lumbar administration of recombinant I2S to enzyme deficient animals reduced the storage of glycosaminoglycans in both superficial and deep brain tissues, with concurrent morphological improvements. The observed patterns of enzyme transport from cerebrospinal fluid to the CNS tissues and the resultant biological activity (a) warrant further investigation of intrathecal delivery of I2S via lumbar catheter as an experimental treatment for the neurological symptoms of Hunter syndrome and (b) may have broader implications for CNS treatment with biopharmaceuticals.
Conflict of interest statement
Figures



References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Laterra J, Goldstein GW. Ventricular organization of the cerebrospinal fluid: blood-brain barrier, brain edema, and hydrocephalus. In: Kandel E, Schwartz JH, Jessel TM, editors. Principles of neural science. New York: McGraw-Hill; 2000. pp. 1288–1301.
-
- Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3:90–105, 151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

