A novel Atoh1 "self-terminating" mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability
- PMID: 22279587
- PMCID: PMC3261193
- DOI: 10.1371/journal.pone.0030358
A novel Atoh1 "self-terminating" mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability
Abstract
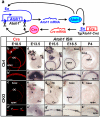
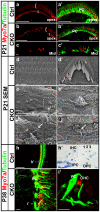
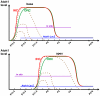
Atonal homolog1 (Atoh1) is a bHLH transcription factor essential for inner ear hair cell differentiation. Targeted expression of Atoh1 at various stages in development can result in hair cell differentiation in the ear. However, the level and duration of Atoh1 expression required for proper hair cell differentiation and maintenance remain unknown. We generated an Atoh1 conditional knockout (CKO) mouse line using Tg(Atoh1-cre), in which the cre expression is driven by an Atoh1 enhancer element that is regulated by Atoh1 protein to "self-terminate" its expression. The mutant mice show transient, limited expression of Atoh1 in all hair cells in the ear. In the organ of Corti, reduction and delayed deletion of Atoh1 result in progressive loss of almost all the inner hair cells and the majority of the outer hair cells within three weeks after birth. The remaining cells express hair cell marker Myo7a and attract nerve fibers, but do not differentiate normal stereocilia bundles. Some Myo7a-positive cells persist in the cochlea into adult stages in the position of outer hair cells, flanked by a single row of pillar cells and two to three rows of disorganized Deiters cells. Gene expression analyses of Atoh1, Barhl1 and Pou4f3, genes required for survival and maturation of hair cells, reveal earlier and higher expression levels in the inner compared to the outer hair cells. Our data show that Atoh1 is crucial for hair cell mechanotransduction development, viability, and maintenance and also suggest that Atoh1 expression level and duration may play a role in inner vs. outer hair cell development. These genetically engineered Atoh1 CKO mice provide a novel model for establishing critical conditions needed to regenerate viable and functional hair cells with Atoh1 therapy.
Conflict of interest statement
Figures



References
-
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. - PubMed
-
- Hildebrand MS, Newton SS, Gubbels SP, Sheffield AM, Kochhar A, et al. Advances in molecular and cellular therapies for hearing loss. Mol Ther. 2008;16:224–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

