Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity
- PMID: 22279596
- PMCID: PMC3261210
- DOI: 10.1371/journal.pone.0030571
Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity
Abstract
Background: In experimental models, hypothalamic inflammation is an early and determining factor in the installation and progression of obesity. Pharmacological and gene-based approaches have proven efficient in restraining inflammation and correcting the obese phenotypes. However, the role of nutrients in the modulation of hypothalamic inflammation is unknown.
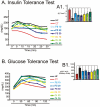
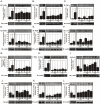
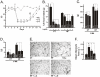
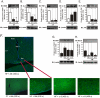
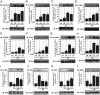
Methodology/principal findings: Here we show that, in a mouse model of diet-induced obesity, partial substitution of the fatty acid component of the diet by flax seed oil (rich in C18:3) or olive oil (rich in C18:1) corrects hypothalamic inflammation, hypothalamic and whole body insulin resistance, and body adiposity. In addition, upon icv injection in obese rats, both ω3 and ω9 pure fatty acids reduce spontaneous food intake and body mass gain. These effects are accompanied by the reversal of functional and molecular hypothalamic resistance to leptin/insulin and increased POMC and CART expressions. In addition, both, ω3 and ω9 fatty acids inhibit the AMPK/ACC pathway and increase CPT1 and SCD1 expression in the hypothalamus. Finally, acute hypothalamic injection of ω3 and ω9 fatty acids activate signal transduction through the recently identified GPR120 unsaturated fatty acid receptor.
Conclusions/significance: Unsaturated fatty acids can act either as nutrients or directly in the hypothalamus, reverting diet-induced inflammation and reducing body adiposity. These data show that, in addition to pharmacological and genetic approaches, nutrients can also be attractive candidates for controlling hypothalamic inflammation in obesity.
Conflict of interest statement
Figures

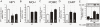

References
-
- Yang L, Hotamisligil GS. Stressing the brain, fattening the body. Cell. 2008;135:20–22. - PubMed
-
- Velloso LA, Araujo EP, de Souza CT. Diet-induced inflammation of the hypothalamus in obesity. Neuroimmunomodulation. 2008;15:189–193. - PubMed
-
- Wisse BE, Schwartz MW. Does hypothalamic inflammation cause obesity? Cell Metab. 2009;10:241–242. - PubMed
-
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

