The role of the Arabidopsis FUSCA3 transcription factor during inhibition of seed germination at high temperature
- PMID: 22279962
- PMCID: PMC3296646
- DOI: 10.1186/1471-2229-12-15
The role of the Arabidopsis FUSCA3 transcription factor during inhibition of seed germination at high temperature
Abstract
Background: Imbibed seeds integrate environmental and endogenous signals to break dormancy and initiate growth under optimal conditions. Seed maturation plays an important role in determining the survival of germinating seeds, for example one of the roles of dormancy is to stagger germination to prevent mass growth under suboptimal conditions. The B3-domain transcription factor FUSCA3 (FUS3) is a master regulator of seed development and an important node in hormonal interaction networks in Arabidopsis thaliana. Its function has been mainly characterized during embryonic development, where FUS3 is highly expressed to promote seed maturation and dormancy by regulating ABA/GA levels.
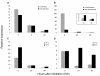
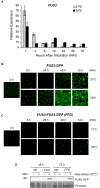
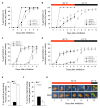
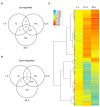
Results: In this study, we present evidence for a role of FUS3 in delaying seed germination at supraoptimal temperatures that would be lethal for the developing seedlings. During seed imbibition at supraoptimal temperature, the FUS3 promoter is reactivated and induces de novo synthesis of FUS3 mRNA, followed by FUS3 protein accumulation. Genetic analysis shows that FUS3 contributes to the delay of seed germination at high temperature. Unlike WT, seeds overexpressing FUS3 (ML1:FUS3-GFP) during imbibition are hypersensitive to high temperature and do not germinate, however, they can fully germinate after recovery at control temperature reaching 90% seedling survival. ML1:FUS3-GFP hypersensitivity to high temperature can be partly recovered in the presence of fluridone, an inhibitor of ABA biosynthesis, suggesting this hypersensitivity is due in part to higher ABA level in this mutant. Transcriptomic analysis shows that WT seeds imbibed at supraoptimal temperature activate seed-specific genes and ABA biosynthetic and signaling genes, while inhibiting genes that promote germination and growth, such as GA biosynthetic and signaling genes.
Conclusion: In this study, we have uncovered a novel function for the master regulator of seed maturation, FUS3, in delaying germination at supraoptimal temperature. Physiologically, this is important since delaying germination has a protective role at high temperature. Transcriptomic analysis of seeds imbibed at supraoptimal temperature reveal that a complex program is in place, which involves not only the regulation of heat and dehydration response genes to adjust cellular functions, but also the activation of seed-specific programs and the inhibition of germination-promoting programs to delay germination.
© 2011 Chiu et al; licensee BioMed Central Ltd.
Figures


Similar articles
-
Overlapping and distinct roles of AKIN10 and FUSCA3 in ABA and sugar signaling during seed germination.Plant Signal Behav. 2012 Oct 1;7(10):1238-42. doi: 10.4161/psb.21549. Epub 2012 Aug 20. Plant Signal Behav. 2012. PMID: 22902692 Free PMC article.
-
ABA-dependent inhibition of the ubiquitin proteasome system during germination at high temperature in Arabidopsis.Plant J. 2016 Dec;88(5):749-761. doi: 10.1111/tpj.13293. Epub 2016 Sep 22. Plant J. 2016. PMID: 27496613
-
Inhibition of FUSCA3 degradation at high temperature is dependent on ABA signaling and is regulated by the ABA/GA ratio.Plant Signal Behav. 2016 Nov;11(11):e1247137. doi: 10.1080/15592324.2016.1247137. Plant Signal Behav. 2016. PMID: 27791466 Free PMC article.
-
LAFL Factors in Seed Development and Phase Transitions.Annu Rev Plant Biol. 2024 Jul;75(1):459-488. doi: 10.1146/annurev-arplant-070623-111458. Epub 2024 Jul 2. Annu Rev Plant Biol. 2024. PMID: 38657282 Review.
-
Transcriptional regulation of plant seed development.Physiol Plant. 2021 Dec;173(4):2013-2025. doi: 10.1111/ppl.13548. Epub 2021 Sep 14. Physiol Plant. 2021. PMID: 34480800 Review.
Cited by
-
DREB2C acts as a transcriptional activator of the thermo tolerance-related phytocystatin 4 (AtCYS4) gene.Transgenic Res. 2014 Feb;23(1):109-23. doi: 10.1007/s11248-013-9735-2. Epub 2013 Jul 19. Transgenic Res. 2014. PMID: 23868510
-
Genome-Wide Identification and Expression Analysis Reveals the B3 Superfamily Involved in Embryogenesis and Hormone Responses in Dimocarpus longan Lour.Int J Mol Sci. 2023 Dec 21;25(1):127. doi: 10.3390/ijms25010127. Int J Mol Sci. 2023. PMID: 38203301 Free PMC article.
-
Overlapping and distinct roles of AKIN10 and FUSCA3 in ABA and sugar signaling during seed germination.Plant Signal Behav. 2012 Oct 1;7(10):1238-42. doi: 10.4161/psb.21549. Epub 2012 Aug 20. Plant Signal Behav. 2012. PMID: 22902692 Free PMC article.
-
High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice.Protoplasma. 2019 Sep;256(5):1217-1227. doi: 10.1007/s00709-019-01354-6. Epub 2019 Apr 18. Protoplasma. 2019. PMID: 31001689
-
Regulation of Seed Germination and Abiotic Stresses by Gibberellins and Abscisic Acid.Front Plant Sci. 2018 Jun 20;9:838. doi: 10.3389/fpls.2018.00838. eCollection 2018. Front Plant Sci. 2018. PMID: 29973944 Free PMC article. Review.
References
-
- Koornneef M, Reuling G, Karssen CM. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant. 1984;61:377–383. doi: 10.1111/j.1399-3054.1984.tb06343.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

