Exploratory experiments on the chemistry of the "glyoxylate scenario": formation of ketosugars from dihydroxyfumarate
- PMID: 22280414
- PMCID: PMC3284196
- DOI: 10.1021/ja211383c
Exploratory experiments on the chemistry of the "glyoxylate scenario": formation of ketosugars from dihydroxyfumarate
Abstract
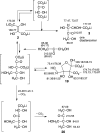
In the context of a "glyoxylate scenario" of primordial metabolism, the reactions of dihydroxyfumarate (DHF) with reactive small molecule aldehydes (e.g., glyoxylate, formaldehyde, glycolaldehyde, and glyceraldehyde) in water were investigated and shown to form dihydroxyacetone, tetrulose, and the two pentuloses, with almost quantitative conversion. The practically clean and selective formation of ketoses in these reactions, with no detectable admixture of aldoses, stands in stark contrast to the formose reaction, where a complex mixture of linear and branched aldoses and ketoses are produced. These results suggest that the reaction of DHF with aldehydes could constitute a reasonable pathway for the formation of carbohydrates and allow for alternative potential prebiotic scenarios to the formose reaction to be considered.
Figures












References
-
- Eschenmoser A. Chem. Biodiversity 2007, 4, 554–573. - PubMed
- Eschenmoser A. Tetrahedron 2007, 63, 12821–12844.
-
- Butlerow A. Justus Liebigs Ann. Chem. 1861, 120, 295–298.
- Butlerow A. C. R. Acad. Sci. 1861, 53, 145–147.
- Fischer E. Ber. Deutsch. Chem. Ges. 1888, 21, 988–992.
- Partridge R. D.; Weiss A. H.; Todd D. Carbohydr. Res. 1972, 24, 29–44.
- Mizuno T.; Weiss A. H. Adv. Carbohydr. Chem. Biochem. 1974, 29, 173–227.
- Decker P.; Schweer H.; Pohlmann R. J. Chromatogr. 1982, 244, 281–291.
- Decker P.; Schweer H. Orig. Life Evol. Biosphere 1984, 14, 335–342.
- Shapiro R. Orig. Life Evol. Biosphere 1988, 18, 71–85. - PubMed
- Schwartz A. W.; de Graaf R. M. J. Mol. Evol. 1993, 36, 101–106.
-
- Loh T.-P.; Wei L.-L.; Feng L.-C. Synlett 1999, 1059–1060.
- Loh T.-P.; Feng L.-C.; Wei L.-L. Tetrahedron 2001, 57, 4231–4236.
-
-
DHF reactions have been investigated for well over a century, starting with the discovery and extensive study of DHF in the late 1800s:
- Bourgoin E. Compt. Rend. 1874, 79, 1053–1054.
- Fenton H. J. H. J. Chem. Soc. Trans. 1894, 65, 899–910.
- Fenton H. J. H. J. Chem. Soc. Trans. 1896, 69, 546–562.
- Fenton H. J. H. J. Chem. Soc. Trans. 1905, 87, 804–818.
- Fenton H. J. H.; Wilks W. A. R. J. Chem. Soc. Trans. 1912, 101, 1570–1582.
-
For X-ray analysis of DHF, see:
- Gupta M. P. J. Am. Chem. Soc. 1953, 75, 6312–6313.
- Gupta M. P.; Gupta N. P. Acta Crystallogr. 1968, B24, 631–636.
- Hartree E. F. J. Am. Chem. Soc. 1953, 75, 6244–6249.
- Goodwin S.; Witkopf B. J. Am. Chem. Soc. 1954, 76, 5012–5014.
- Yalpani M.; Wilke G. Chem. Ber. 1985, 118, 661–669.
- Kemp D. S.; Bowen B. J. Tetrahedron Lett. 1988, 29, 5077–5080.
- Jung M. E.; Blum R. E.; Gaede B. J.; Gisler M. R. Heterocycles 1989, 28, 93–97.
- Reithel F. J.; West E. S. J. Am. Chem. Soc. 1948, 70, 898–900. - PubMed
-
-
- Fenton H. J. H. J. Chem. Soc. Trans. 1895, 67, 774–780.
- Fanke W.; Brathun G. Justus Liebigs Ann. Chem. 1931, 487, 1–52.
- Hay R. W.; Harvie S. Aust. J. Chem. 1965, 18, 1197–1209.
- Fleury M. Compt. Rend. 1965, 260, 5563–5566.
- Wong C. H.; Whitesides G. M. J. Am. Chem. Soc. 1983, 105, 5599–5603.
- Dazou D.; Karabinas P.; Jannakoudkis D. J. Electroanal. Chem. 1984, 176, 225–234.
- Garcia B.; Ruiz R.; Leal J. M. J. Phys. Chem. A 2008, 112, 4921–4928. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

