Mucoadhesive fenretinide patches for site-specific chemoprevention of oral cancer: enhancement of oral mucosal permeation of fenretinide by coincorporation of propylene glycol and menthol
- PMID: 22280430
- PMCID: PMC3687357
- DOI: 10.1021/mp200655k
Mucoadhesive fenretinide patches for site-specific chemoprevention of oral cancer: enhancement of oral mucosal permeation of fenretinide by coincorporation of propylene glycol and menthol
Abstract
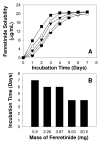
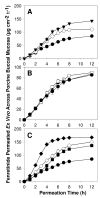
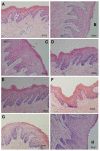
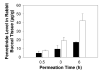
The objective of this study was to enhance oral mucosal permeation of fenretinide by coincorporation of propylene glycol (PG) and menthol in fenretinide/Eudragit RL PO mucoadhesive patches. Fenretinide is an extremely hydrophobic chemopreventive compound with poor tissue permeability. Coincorporation of 5-10 wt % PG (mean J(s) = 16-23 μg cm⁻² h⁻¹; 158-171 μg of fenretinide/g of tissue) or 1-10 wt % PG + 5 wt % menthol (mean J(s) = 18-40 μg cm⁻² h⁻¹; 172-241 μg of fenretinide/g of tissue) in fenretinide/Eudragit RL PO patches led to significant ex vivo fenretinide permeation enhancement (p < 0.001). Addition of PG above 2.5 wt % in the patch resulted in significant cellular swelling in the buccal mucosal tissues. These alterations were ameliorated by combining both enhancers and reducing PG level. After buccal administration of patches in rabbits, in vivo permeation of fenretinide across the oral mucosa was greater (∼43 μg fenretinide/g tissue) from patches that contained optimized permeation enhancer content (2.5 wt % PG + 5 wt % menthol) relative to permeation obtained from enhancer-free patch (∼17 μg fenretinide/g tissue) (p < 0.001). In vitro and in vivo release of fenretinide from patch was not significantly increased by coincorporation of permeation enhancers, indicating that mass transfer across the tissue, and not the patch, largely determined the permeation rate control in vivo. As a result of its improved permeation and its lack of deleterious local effects, the mucoadhesive fenretinide patch coincorporated with 2.5 wt % PG + 5 wt % menthol represents an important step in the further preclinical evaluation of oral site-specific chemoprevention strategies with fenretinide.
Figures


References
-
- Lefebvre JL. Current Clinical Outcomes Demand New Treatment Options for SCCHN. Ann. Oncol. 2005;16:VI7–VI12. - PubMed
-
- Dent J. Molecular Pathogenesis of Oral Squamous Cell Carcinoma: Implications for Therapy. J. Dent. Res. 2008;87:191–191. - PubMed
-
- Forastiere AA. Head and Neck Cancer: Overview of Recent Dvelopments and Future Directions. Semin. Oncol. 2000;27:1–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

