Convective tissue movements play a major role in avian endocardial morphogenesis
- PMID: 22280991
- PMCID: PMC3288244
- DOI: 10.1016/j.ydbio.2011.12.036
Convective tissue movements play a major role in avian endocardial morphogenesis
Abstract
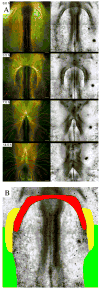
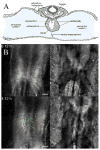
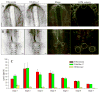
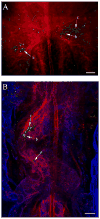
Endocardial cells play a critical role in cardiac development and function, forming the innermost layer of the early (tubular) heart, separated from the myocardium by extracellular matrix (ECM). However, knowledge is limited regarding the interactions of cardiac progenitors and surrounding ECM during dramatic tissue rearrangements and concomitant cellular repositioning events that underlie endocardial morphogenesis. By analyzing the movements of immunolabeled ECM components (fibronectin, fibrillin-2) and TIE1 positive endocardial progenitors in time-lapse recordings of quail embryonic development, we demonstrate that the transformation of the primary heart field within the anterior lateral plate mesoderm (LPM) into a tubular heart involves the precise co-movement of primordial endocardial cells with the surrounding ECM. Thus, the ECM of the tubular heart contains filaments that were associated with the anterior LPM at earlier developmental stages. Moreover, endocardial cells exhibit surprisingly little directed active motility, that is, sustained directed movements relative to the surrounding ECM microenvironment. These findings point to the importance of large-scale tissue movements that convect cells to the appropriate positions during cardiac organogenesis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




References
-
- Alexander J, Stainier DYR. Mutations affecting cardiac development in zebrafish. In: Harvey RP, Rosenthal N, editors. Heart Development. San Diego, London, Boston, New York, Sydney, Tokyo, Toronto: Academic Press; 1999. pp. 91–110.
-
- Bellairs R, Osmond M. Atlas of Chick Development. 2. Academic Press; 2005.
-
- Bowers SL, Baudino TA. Laying the groundwork for growth: Cell-cell and cell-ECM interactions in cardiovascular development. Birth Defects Res C Embryo Today. 2010;90(1):1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

