Assessment of differential pharmacodynamic effects using optical coherence tomography in neovascular age-related macular degeneration
- PMID: 22281826
- PMCID: PMC3339900
- DOI: 10.1167/iovs.11-8130
Assessment of differential pharmacodynamic effects using optical coherence tomography in neovascular age-related macular degeneration
Abstract
Purpose: To use novel OCT parameters in assessing the differential pharmacodynamic effects of bevacizumab (Avastin; Genentech, South San Francisco, CA), pegaptanib (Macugen; OSI Pharmaceuticals, New York, NY), and verteporfin photodynamic therapy (PDT; Novartis, Basel, Switzerland) in a recently completed phase III/IV clinical trial.
Methods: Data from 122 patients participating in the Avastin (Bevacizumab) for Choroidal Neovascularization (ABC) trial, were evaluated. OCT scans were analyzed with custom software. Changes in the volume of the neurosensory retina, amount of subretinal fluid (SRF), pigment epithelium detachment (PED), and subretinal tissue (SRT), were calculated over the 54-week trial period.
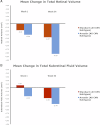
Results: Reductions in retinal edema were more than twice as great from bevacizumab as from pegaptanib (-0.82 mm³ vs. -0.31 mm³), whereas SRF reduction was more than three times greater (-0.54 mm³ vs. -0.15 mm³. Both bevacizumab and pegaptanib led to rapid reductions in SRT; however, in those receiving pegaptanib, these improvements were not maintained (at week 54, -0.22 mm³ vs. +0.18 mm³). Acute increases in SRF were seen 1 week after PDT (+0.36 mm³) and, across all treatment groups, PED volume tended to remain unchanged or to regress only slowly.
Conclusions: In clinical trials, quantitative OCT subanalysis increases the amount of clinically useful information that can be obtained from OCT images. In the emerging era of neovascular AMD therapeutics, the capacity of OCT to provide such detailed pharmacodynamic information in a noninvasive manner is likely to attain increased importance. In future comparative studies, evaluation of SRT may highlight differential effects on vascular proliferation, whereas measurement of PED volume may be useful for the estimation of retinal and subretinal pigment epithelium (RPE) therapeutic penetration. (ClinicalTrials.gov number, ISRCTN83325075.).
Figures




References
-
- Bressler NM. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology. 2009;116:S15–S23 - PubMed
-
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617 - PubMed
-
- Tufail AT, Patel PJ, Egan C, et al. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ. 2010;340:c2459. - PubMed
-
- Holz FG, Amoaku W, Donate J, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118:663–671 - PubMed
-
- Lalwani G, Rosenfeld P, Fung A, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148:43–58.e1 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

