Bupropion for smoking cessation in African American light smokers: a randomized controlled trial
- PMID: 22282543
- PMCID: PMC3283533
- DOI: 10.1093/jnci/djr513
Bupropion for smoking cessation in African American light smokers: a randomized controlled trial
Abstract
Background: Previous research demonstrated the efficacy of sustained release bupropion (bupropion SR) for smoking cessation in whites as well as moderate to heavy (≥10 cigarettes per day [CPD]) African American smokers. We evaluated whether bupropion SR was effective for smoking cessation among African American light smokers (≤10 CPD).
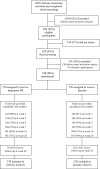
Methods: A randomized, double-blind placebo-controlled trial was conducted from December 27, 2007, to May 13, 2010. All participants were African American light smokers (≤10 CPD), aged 18 years or older. Participants were randomly assigned to receive 300 mg bupropion SR (150 mg once daily for 3 days and then 150 mg twice daily) (n = 270 participants) or placebo (n = 270 participants) for 7 weeks, and up to six sessions of health education counseling. Serum cotinine was measured at baseline (week 0). The primary outcome was salivary cotinine-verified 7-day point prevalence smoking abstinence at week 26; a cut point of 15 ng/mL differentiated smokers from nonsmokers. Salivary cotinine-verified smoking abstinence at end of medication treatment at week 7 was also examined. Odds ratios (OR) for smoking abstinence and 95% confidence intervals (CIs) were calculated using logistic regression models. All statistical tests were two-sided.
Results: Participants at baseline visit (week 0) smoked an average of 8.0 CPD and had a mean serum cotinine level of 275.8 ng/mL (SD = 155.8 ng/mL); most used menthol cigarettes (83.7%) and smoked within 30 minutes of waking (72.2%). After imputing those lost to follow-up as smokers, no statistically significant difference in long-term smoking abstinence rates at week 26 was observed between bupropion SR and placebo groups (13.3% vs 10.0%, OR = 1.39, 95% CI = 0.82 to 2.35, P = .23). Cotinine-verified smoking abstinence rate at end of medication week 7 was higher in the bupropion SR vs placebo group (23.7% vs 9.6%, OR = 2.92, 95% CI = 1.78 to 4.77, P < .001).
Conclusions: Bupropion SR was effective in promoting smoking cessation during the medication phase of treatment but showed no effect on long-term smoking cessation among African American light smokers. More research is needed to identify strategies for sustaining abstinence among African American light smokers.
Figures


Comment in
-
Enhancing the effectiveness of smoking cessation interventions: a cancer prevention imperative.J Natl Cancer Inst. 2012 Feb 22;104(4):260-2. doi: 10.1093/jnci/djr558. Epub 2012 Jan 25. J Natl Cancer Inst. 2012. PMID: 22282541 Free PMC article. No abstract available.
References
-
- American Cancer Society Cancer Facts and Figures 2009. Atlanta, GA: 2009. American Cancer Society;
-
- Schoenborn CA, Adams PF, Barnes PM, Vickerie JL, Schiller JS. Health behaviors of adults: United States, 1999-2001. Vital Health Stat 10. 2004;219:1–79. - PubMed
-
- CDC. Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1227–1232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

